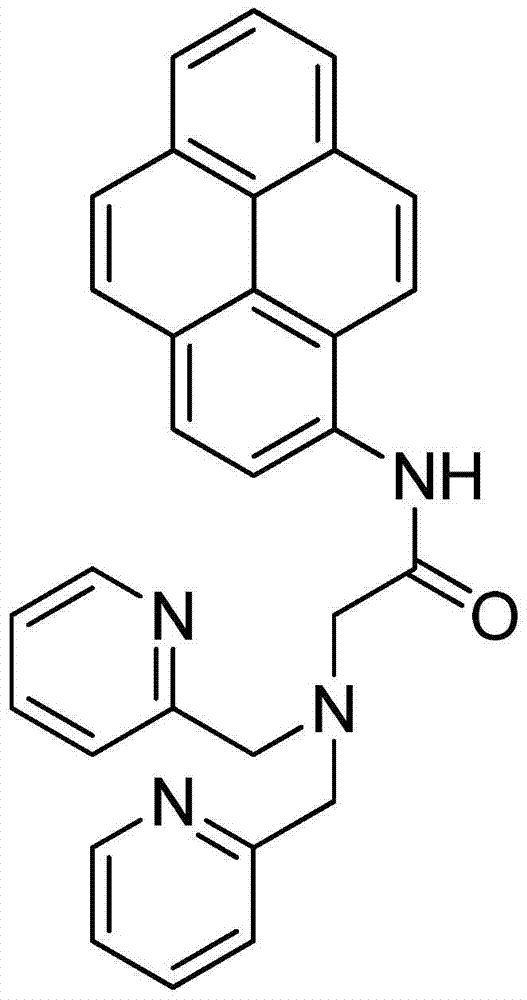
Cadmium-ion fluorescence probe based on pyrene exciplex, and preparation method and application thereof
A technology of fluorescent probes and cadmium ions, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials. simple route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
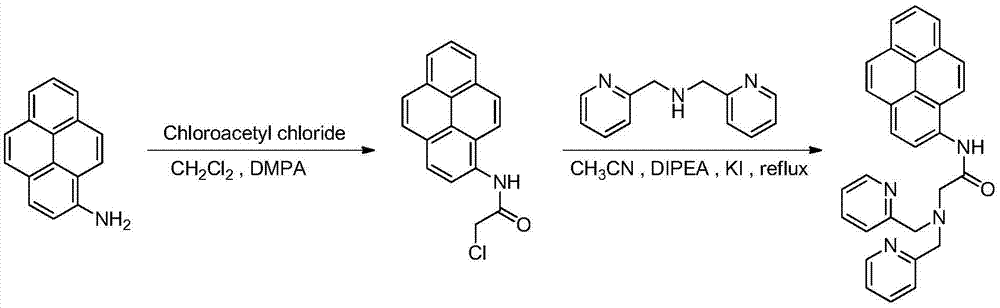
[0026] Example 1: Exciplex-type cadmium ion fluorescent probe, the basic synthesis process is as follows:
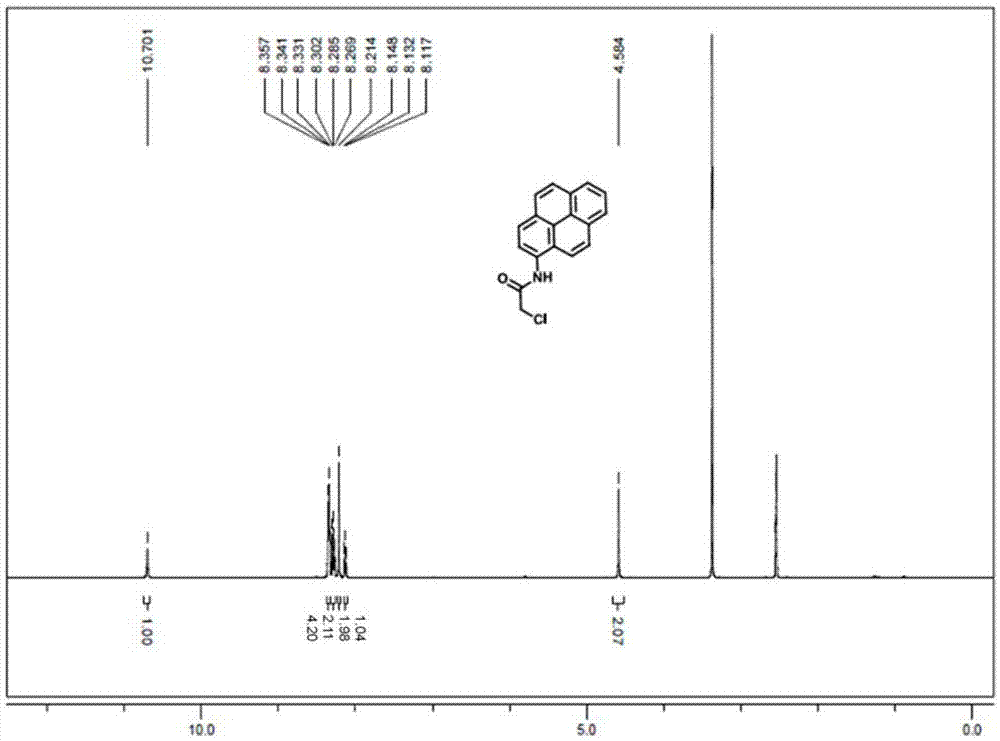
[0027] 1) Synthesis of 1-(2-chloroacetyl)pyrene: add 1g of 1-aminopyrene to a one-necked bottle, add 100mL of CH 2 Cl 2 As a solvent, 0.9 g of DMAP was added, and 0.43 mL of chloroacetyl chloride was slowly added dropwise thereto under ice-cooling conditions. Slowly rose to room temperature and stirred for 6h. After the reaction was completed, the solvent was evaporated by rotary evaporation, and passed through a silica gel column, eluting with petroleum ether:ethyl acetate=10:1. The eluent containing the product was collected by spin-drying, and the product was a white solid with a yield of 74%. The H NMR spectrum and carbon spectrum of the intermediate are as follows: image 3 , Figure 4 shown.
[0028] 2) Synthesis of the probe: Add 0.63g of 1-(2-chloroacetyl)pyrene to a one-necked bottle, add 150mL of acetonitrile as a solvent, then add 0.38g of KI and 6mL of ...
Embodiment 2
[0030] Embodiment 2: The fluorescent probe prepared in embodiment 1 is to the response situation of cadmium ion determination
[0031] The probe prepared in Example 1 was dissolved in CH 3 CN solution, added to CH 3 CN:H 2 In O(1:19, v / v) mixed solvent, the concentration of the probe was prepared to be 10 -5 M solution, test its fluorescence spectrum changes.
[0032] Figure 7 When the concentration of the probe is 10 μM, when the concentration of cadmium ions is 5 μM, the fluorescence spectrum is significantly enhanced at 490 nm, indicating that after the two molecular probes are combined with a cadmium ion in a 2:1 manner, the two pyrene fluorophores are close to each other. Exciplex fluorescence peaks are generated. Its excitation wavelength is 360nm.
Embodiment 3
[0033] Embodiment 3: the fluorescent probe prepared in embodiment 1 and cadmium ion in CH 3 Complexation ratio in CN solution
[0034] The CH that the probe prepared in Example 1 is dissolved in 3 In the CN solution, the probe concentration was prepared to be 10 -5 The solution of M, the change of the fluorescence spectrum after the test probe is complexed with different metal ions. The added metal ions are 3eq of the probe concentration, which are Zn-containing 2+ , Fe 3+ , Cr 3+ , Ni2+ ,Co 2+ , Pd 2+ , Ag + , Cu 2+ , Hg 2+ , Mn 2+ , Cu + , Fe 2+ , Cd 2+ , Na + , Pb 2+ , Ca 2+ , Pt 2+ , Mg 2+ , K + Aqueous perchlorate solution. The result is as Figure 8 , indicating that the addition of excess Cd 2+ , will make the probe with Cd 2+ A 1:1 complexation is formed, resulting in a certain degree of quenching of the monomer fluorescence peak.
[0035] Keep the sum of the concentration of probe and cadmium ion at 10 μM in CH 3 CN: H2O=5:95(v / v) Solvent resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com