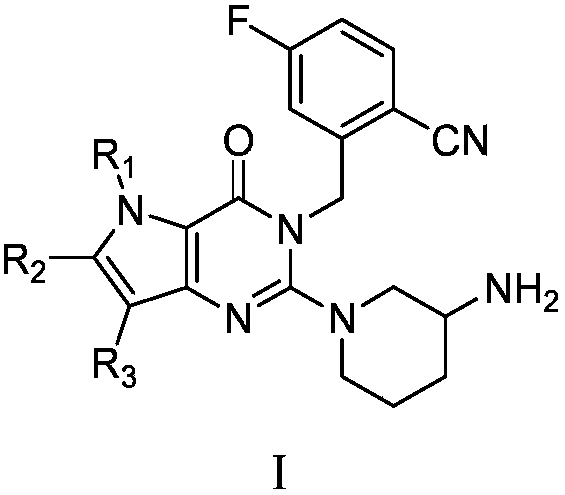
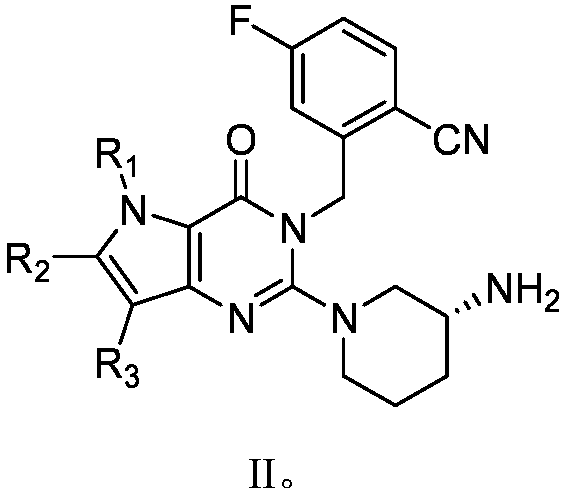
Pyrrolopyrimidine ketone compound and preparation method and application thereof
A technology of pyrrolopyrimidones and compounds, applied in the field of pyrrolopyrimidones and their preparation, capable of solving problems such as cardiovascular pathological reactions, hypoglycemia, and β-cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1. Synthesis of Compound 1.
[0115]
[0116] Proceed according to the following synthetic route:
[0117]
[0118] 1) Synthesis of compound 1-1:
[0119] Add 520ml of concentrated sulfuric acid into a 100mL eggplant-shaped bottle, add 140g of 6-methyluracil in batches under ice-cooling, and keep the temperature not exceeding 40°C. After it is completely dissolved, slowly add 104ml of fuming nitric acid dropwise, keeping the temperature not exceeding 15°C. After the dropwise addition was completed, after stirring at room temperature for 30 min, the reaction solution was poured into crushed ice, and solids were washed out. After suction filtration, the filter cake was washed with water and acetone and then dried to obtain the target compound 1-1.
[0120] 1H-NMR (400 MHz, DMSO-d6): δ 11.79 (1H, s), 11.76 (1H, s), 2.28 (3H, s).
[0121] 2) Synthesis of compound 1-2:
[0122] Add 119g of compound 1-1 and 400mL of N,N-dimethylformamide into a 1000mL two-nec...
Embodiment 2
[0142] Embodiment 2. Synthesis of compound 2
[0143]
[0144] Proceed according to the following synthetic route:
[0145]
[0146] 1) Synthesis of compound 2-1:
[0147] Compound 1-7 (303 mg, 1.0 mmol) was dissolved in 20 mL of THF, 60% sodium hydrogen (48 mg, 1.2 mmol) was added under nitrogen protection at 0°C, stirred until no gas was generated, and methanesulfonyl chloride (137 mg, 1.1 mmol), gradually raised to room temperature and stirred for 5 hours, the reaction solution was added with 50mL of water, then extracted with 3×80mL of ethyl acetate, the organic layers were combined, washed successively with saturated aqueous sodium bicarbonate solution, water and saturated brine, anhydrous sodium sulfate Drying, separation by flash column chromatography to obtain compound 2-1 with a yield of 88%;
[0148] MS: 381.0 [M+H + ].
[0149] 2) Synthesis of compound 2:
[0150] Compound 2-1 obtained in the above steps was used as a raw material, and compound 2 was synt...
Embodiment 3
[0152] Embodiment 3. Synthesis of compound 3
[0153]
[0154] Proceed according to the following synthetic route:
[0155]
[0156] Using compound 1-7 as raw material, 2-cyano-5-fluorobenzyl bromide was used instead of methanesulfonyl chloride, referring to the synthesis method of Example 2, compound 3 was synthesized with a yield of 53%;
[0157] MS: 500.2 [M+H + ].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com