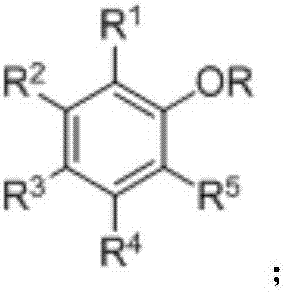
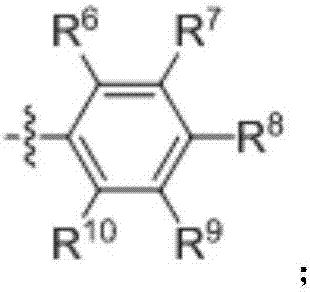
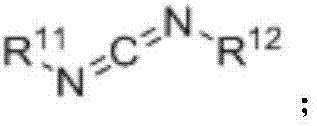Ether bond breakage method for phenylalkyl ethers
A technology of phenyl alkyl ether and ether bond, which is applied in the field of intermediate synthesis of medicines and chemical raw materials, can solve the problems such as low yield of eugenol demethylation reaction, and achieve the effect of wide application range and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (eugenol demethylation)
[0035]
[0036] Add aluminum triiodide (2.247g), acetonitrile (40ml), DCC (0.618g) and eugenol (0.818g) respectively in a 100ml eggplant-shaped bottle, heat to 80°C, stop stirring after 18 hours of reaction, cool to After room temperature, add 2mol / L dilute hydrochloric acid (50ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, wash with saturated sodium thiosulfate aqueous solution (10ml), and then wash with saturated Wash with brine (10ml), dry over anhydrous magnesium sulfate, filter, evaporate the filtrate to dryness with a rotary evaporator, and purify the residue by flash column chromatography (eluent: ethyl acetate / petroleum ether=1:4, volume ratio) , to obtain 0.721 g of 4-allyl catechol (white waxy solid, yield 96%).
[0037] 1 H NMR (400MHz, CDCl 3 )δ6.80(d, J=8Hz, 1H), 6.72(d, J=2Hz, 1H), 6.63(dd, J 1 =8Hz,J 2 =2Hz,1H),6.10(brs,2H),5.92(ddt,J 1 =17.2H...
Embodiment 2
[0039] Embodiment 2 (eugenol demethylation)
[0040] Add aluminum triiodide (2.252g), acetonitrile (40ml), N,N'-dicyclohexylcarbodiimide DCC (1.036g) and eugenol (0.818g) respectively in a 100ml eggplant-shaped bottle, and heat to 80°C, stop stirring after 18 hours of reaction, cool to room temperature, add 2mol / L dilute hydrochloric acid (50ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, and first use thio Wash with saturated aqueous sodium sulfate (10ml), then with saturated brine (10ml), dry over anhydrous magnesium sulfate, filter, and evaporate the filtrate to dryness with a rotary evaporator, and the residue is subjected to flash column chromatography (eluent is ethyl acetate / petroleum ether=1:4, volume ratio) to obtain 0.723 g of 4-allyl catechol (white waxy solid, yield 96%).
Embodiment 3
[0041] Embodiment 3 (eugenol demethylation)
[0042] Add aluminum triiodide (2.247g), acetonitrile (40ml), DCC (1.549g) and eugenol (0.818g) respectively in a 100ml eggplant-shaped bottle, heat to 80 ° C, stop stirring after 18 hours of reaction, cool to After room temperature, add 2mol / L dilute hydrochloric acid (50ml) into the eggplant-shaped bottle to acidify, extract with ethyl acetate (50ml×3), combine the organic phases, wash with saturated aqueous sodium thiosulfate (10ml), and then wash with saturated Wash with brine (10ml), dry over anhydrous magnesium sulfate, filter, evaporate the filtrate to dryness with a rotary evaporator, and purify the residue by flash column chromatography (eluent: ethyl acetate / petroleum ether=1:4, volume ratio) , to obtain 0.684 g of 4-allyl catechol (white waxy solid, yield 91%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com