Inhibitor and application thereof in inhibiting chitinase and hexosaminidase activity
A technology of hexosaminidase and chitinase, applied in application, biocides, insecticides, etc., can solve the problem of no report on the biological activity of glycosyl hydrolases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Using chitinase OfChi-h and hexosaminidase OfHex1 as targets, 556 microbial secondary metabolites were screened for inhibitors. Specific steps are as follows:
[0022] Positive control: Set up 2 parallel positive controls. Under the condition of 30℃ reaction temperature and 100μL reaction system, 2nmol / L glycosyl hydrolase and 50μmol / L substrate (OfChi-h: MU-(GlcNAc) 2 ; OfHex1: MU-GlcNAc) was incubated in 20mmol / L pH6.0 phosphate buffer for 30min, then 100μL 0.5mol / L sodium carbonate solution was added to terminate the reaction, and the reaction solution was excited with 360nm wavelength excitation light to measure 450nm The absorbance value at the wavelength.
[0023] Experimental group: set up 3 parallel experimental groups. Under the condition of 30℃ reaction temperature and 100μL reaction system, 2nmol / L glycosyl hydrolase and 50μmol / L substrate (OfChi-h: MU-(GlcNAc) 2 ; OfHex1: MU-GlcNAc) and 13ppm compound were incubated in 20mmol / L pH 6.0 phosphate buffer fo...
Embodiment 2
[0028] 1) Absolute configuration identification of compound phlegmacin B1
[0029] Take 500 μL of 100% methanol and add it into the cuvette for circular dichroism scanning, and the result is used as a negative control. The compound phlegmacin B1 was dissolved in 100% methanol to make the final concentration 0.7 μM, and 500 μL was added to a cuvette, and its circular dichroism spectrum was measured in a circular dichroism instrument, and the results were as follows figure 1 As shown in A. The circular dichroism chromatogram measured by the experiment is compared with the circular dichroism chromatogram in the document "Biosynthesisand Stereochemistry of Phlegmacin-Type Fungal Pigments" to obtain the absolute configuration of compound phlegmacin B1, such as figure 1 b. Experimental parameters: optical path 2mm; scanning wavelength 190-350nm; speed 1nm / s; repetition times 3 times.
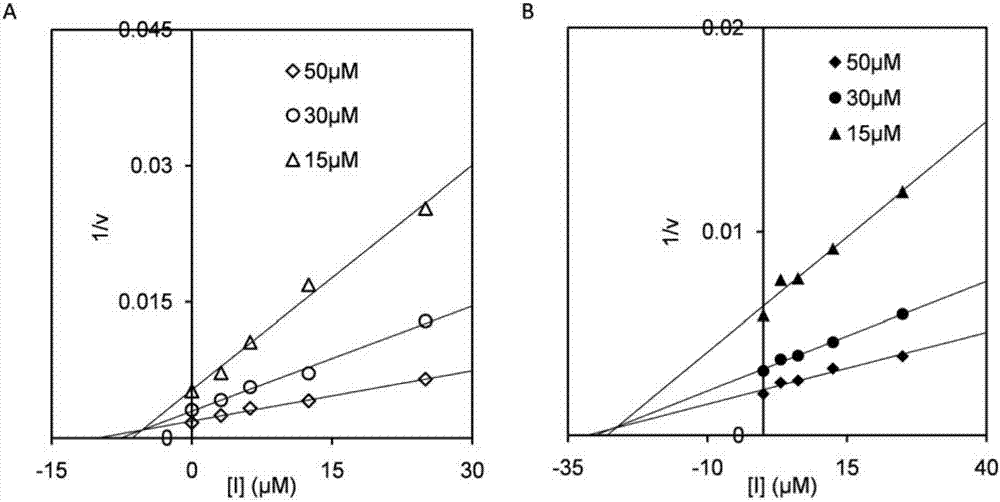
[0030] 2) Inhibition constant K i determination
[0031] OfChi-h: MU-(GlcNAc) 2 As substrate...
Embodiment 3
[0038] The specific steps of the insecticidal activity evaluation of compound phlegmacin B1 are as follows:
[0039] In the experiment, healthy larvae on the fourth day of the fifth instar were selected as experimental materials, and a control group and an experimental group were set up. Compound phlegmacin B1 was dissolved in 5% DMSO at a concentration of 0.5 μM. The larvae of the control group were injected with 2 μL of 5% DMSO, and the larvae of the experimental group were injected with 2 μL of the compound phlegmacin B1 at a concentration of 0.5 μM. The injected larvae were cultured at 26°C, with a relative humidity of 70%-90%, 16 hours of light per day, and 8 hours of darkness until all of them pupated. During this period, normal larvae, dead larvae, normal pupae and abnormal pupae Quantities and phenotypes were counted. The statistical results are plotted as Figure 4 , where A is the control group, B is the experimental group, and C is the phenotype. The results sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com