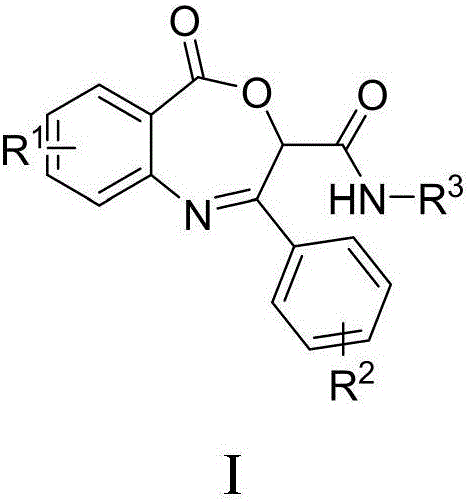
Benzoxazepin bacteriostats and synthetic method thereof
A technology of benzoxanthine and a bacteriostatic agent is applied in the application field of active ingredients to achieve the effects of low preparation cost, high reaction efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
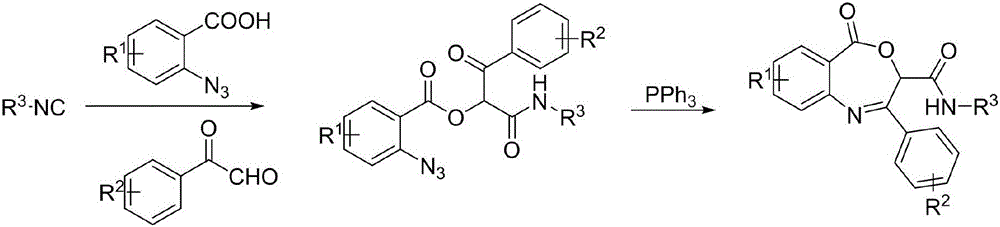
[0027] A synthetic N-(tert-butyl)-2-(4-chlorophenyl)-5-oxo-3,5-dihydrobenzo[e][1,4]oxazepine-3-carboxamide method, including the following experimental steps:
[0028] Weigh o-azidobenzoic acid 1 (0.16g, 1mmol), weigh p-chloroacetophenone aldehyde 2 (0.17g, 1mmol) and tert-butylisonitrile 3 (0.08g, 1mmol) and add them to 5ml of dichloromethane, Placed at room temperature and reacted for 24 hours, azidobenzoate derivatives can be generated; the reaction solution was not separated, and triphenylphosphine (3.15g, 1.2mmol) was added, and intramolecular Wittig reaction; after the reaction is completed, the target compound 4a is obtained by column chromatography separation and purification through a silica gel column:
[0029]
[0030] Yield: 86%
[0031]
[0032] 1 HNMR (CDCl 3 ,600MHz)δ(ppm)7.98(d,J=6.6Hz,1H,Ar-H),7.76-7.69(m,3H,Ar-H),7.46-7.39(m,4H,Ar-H),6.79 (s,1H,NH),5.16(s,1H,CH),1.38(s,9H,CH 3 ).
[0033] HRMS Calculated for [C 20 h 19 ClN 2 o 3 +H]+:371.1162...
Embodiment 2
[0035] A method for synthesizing (4-bromophenyl)-N-(tert-butyl)-5-oxo-3,5-dihydrobenzo[e][1,4]oxazepine-3-carboxamide , including the following experimental steps:
[0036] Weigh o-azidobenzoic acid 1 (0.16g, 1mmol), weigh p-bromoacetophenone aldehyde 2 (0.21g, 1mmol) and tert-butylisonitrile 3 (0.08g, 1mmol) and add them to 5ml of dichloromethane, Placed at room temperature and reacted for 24 hours, azidobenzoate derivatives can be generated; the reaction solution was not separated, and triphenylphosphine (3.15g, 1.2mmol) was added, and intramolecular Wittig reaction; after the reaction is completed, the target compound 4b is obtained by column chromatography separation and purification through a silica gel column:
[0037] Yield: 83%
[0038]
[0039] 1 H NMR (CDCl 3 ,600MHz)δ(ppm)7.97(d,J=7.8Hz,1H,Ar-H),7.70-7.56(m,5H,Ar-H),7.45-7.38(m,2H,Ar-H),6.77 (s,1H,NH),5.16(s,1H,CH),1.37(s,9H,CH 3 ).
[0040] HRMS Calculated for [C 20 h 19 BrN 2 o 3 +H]+: 415.0657, Fo...
Embodiment 3
[0042] A method for synthesizing (4-chlorophenyl)-N-cyclohexyl-5-oxo-3,5-dihydrobenzo[e][1,4]oxazepine-3-carboxamide, comprising the following Experimental steps:
[0043] Weigh o-azidobenzoic acid 1 (0.16g, 1mmol), weigh p-chloroacetophenone aldehyde 2 (0.17g, 1mmol) and cyclohexylisocyanide 3 (0.11g, 1mmol) and add them to 5ml of dichloromethane , placed at room temperature and reacted for 24 hours to generate azidobenzoate derivatives; the reaction solution was not separated, and triphenylphosphine (3.15g, 1.2mmol) was added, and molecular Inner Wittig reaction; the residue was separated and purified by silica gel column chromatography to obtain the target compound 4c:
[0044] Yield: 79%
[0045]
[0046] 1 H NMR (CDCl 3 ,600MHz)δ(ppm)7.98(d,J=7.8Hz,1H,Ar-H),7.74-7.69(m,3H,Ar-H),7.46-7.40(m,4H,Ar-H),6.91 (s,1H,NH),5.24(s,1H,CH),3.79(s,1H,CH),1.97-1.74(m,4H,CH 2 ),1.34-1.17(m,6H,CH 2 ).
[0047] HRMS Calculated for [C 22 h 21 ClN 2 o3 +H]+: 397.1319, Found: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com