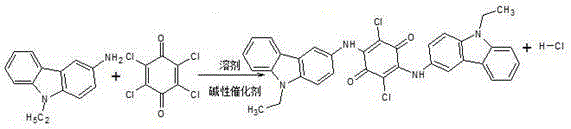
Permanent violet RL intermediate condensation product synthesis process
A synthesis process, a technology for permanent violet, applied in the field of synthetic technology of permanent violet RL intermediate condensate, can solve the problems of difficult washing of side reactants, affecting product quality, multiple raw materials, etc., to reduce impurity content, process stability, The effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis process of permanent violet RL intermediate condensate includes the following steps:
[0024] S1. Alkylation reaction: Add lye and chlorobenzene with a mass concentration of 43% in the reaction kettle, and after stirring for 10 minutes, add carbazole and benzyltriethylammonium bromide; divide bromoethane into 3 equal parts , adjust the temperature in the reactor to 20~25℃ under constant stirring, slowly add the first bromoethane dropwise, after stirring for 15min, slowly add the second bromoethane, stir for 15min, then adjust the temperature to 30~ Slowly add the third portion of bromoethane at 36°C, stir, cover and seal for 20 minutes, then adjust the temperature to 60~68°C, and react for 2~3 hours; - Ethylcarbazole;
[0025] S2. Nitration reaction: Add chlorobenzene and N-ethylcarbazole into the reaction kettle, and slowly add nitric acid with a mass concentration of 35%~36.5% under constant stirring, and the dropping time is 2~3.5h. After completion, ...
Embodiment 2
[0040]The process steps of this embodiment and embodiment 1 are the same, the difference is (2) in step S4 condensation reaction: adjust the temperature to 40 ~ 45 ° C, then open the lid of the kettle, put in 64 kg of sodium carbonate (industrial grade) with a content of 98%, The feeding time is controlled within 10-25 minutes. Among them, the weight ratio of materials 3-amino-N-ethylcarbazole: ethanol: chloranil: sodium carbonate = 1: (18~22): (0.58~0.60): (0.32~0.34).
Embodiment 3
[0042] The process steps of this embodiment are the same as that of Embodiment 1, the difference lies in the difference of step S1:
[0043] S1. Alkylation reaction: Add lye and dichlorobenzene with a mass concentration of 45% in the reaction kettle, and after stirring for 10 minutes, add carbazole and benzyltriethylammonium chloride; divide bromoethane into equal amounts of 3 part, under constant stirring, adjust the temperature in the reactor to 20~25°C, slowly add the first part of bromoethane dropwise, after stirring for 15 minutes, slowly add the second part of bromoethane, stir for 15 minutes, then adjust the temperature to 30 ~36°C, slowly add the third portion of bromoethane, stir, cover and seal for 20 minutes, then adjust the temperature to 60~68°C, and react for 2~3 hours; after the reaction, dichlorobenzene is recovered by distillation, and sodium bromide is separated by standing and N-ethylcarbazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com