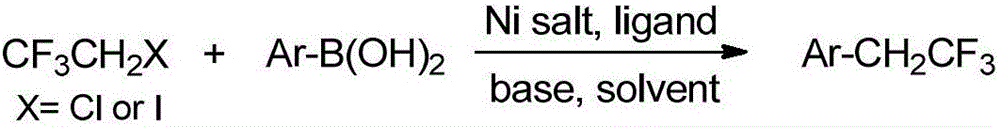Preparation method of trifluoroethyl-substituted aromatic compound
An aromatic compound, trifluoroethyl technology, applied in the field of preparation of trifluoroethyl-substituted aromatic compounds, can solve the problems of restricting industrial production safety, high risk of reaction reagents, high catalyst cost, etc., and achieve easy expansion of reaction scale, Low cost of raw materials and catalysts, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
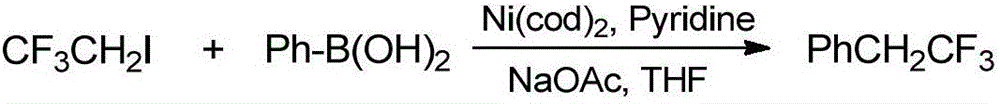
[0023] Preparation of compound 2,2,2-trifluoroethylbenzene
[0024]
[0025] Under a nitrogen atmosphere, phenylboronic acid (18.29g, 150mmol) and anhydrous sodium acetate (24.61g, 300mmol) were sequentially added to the sealed tube, and the solvent tetrahydrofuran (300mL) was added and stirred evenly, and then 2-iodo-1,1 , 1-trifluoroethane (20.99g, 100mmol), pyridine (1.58g, 20mmol) and Ni(cod) 2 (2.75g, 10mol), after sealing, stirred and reacted in an oil bath at 80°C for 12 hours, cooled the reaction solution to room temperature, added benzotrifluoride, and monitored the end point of the reaction using the fluorine spectrum internal standard method, and determined that the crude yield of the reaction was 32 % (the product 2,2,2-trifluoroethylbenzene has a low boiling point, and the yield was calculated without purification and separation).
Embodiment 2
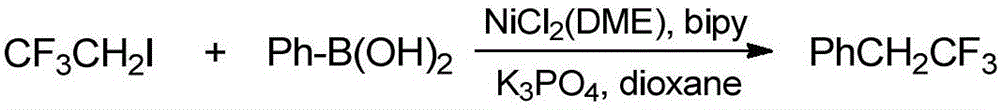
[0027] Preparation of compound 2,2,2-trifluoroethylbenzene
[0028]
[0029] Under nitrogen atmosphere, phenylboronic acid (15.85g, 130mmol) and anhydrous potassium phosphate (74.29g, 350mmol) were successively added into the sealed tube, and the solvent dioxane (250mL) was added and stirred evenly, and then 2-iodo- 1,1,1-Trifluoroethane (20.99g, 100mmol), 2,2'-bipyridine (1.56g, 10mmol) and NiCl 2 (DME) (2.20g, 10mmol), after sealing, stirred and reacted in an oil bath at 80°C for 12 hours, cooled the reaction solution to room temperature, added benzotrifluoride, and monitored the end point of the reaction using the internal standard method of fluorine spectroscopy, and determined the crude product of the reaction. The rate is 60%.
Embodiment 3
[0031] Preparation of compound 2,2,2-trifluoroethylbenzene
[0032]
[0033] Under nitrogen atmosphere, add phenylboronic acid (15.85g, 130mmol) and anhydrous potassium phosphate (42.45g, 200mmol) in sequence in the sealed tube, add solvent toluene (200mL) and stir evenly, then add 2-iodo-1,1 , 1-trifluoroethane (20.99g, 100mmol), α,α,α-terpyridine (4.34g, 10mmol) and Ni(acac) 2 (2.57g, 10mmol), after sealing, stir and react in an oil bath at 80°C for 18 hours, cool the reaction solution to room temperature, add trifluorotoluene, use the fluorine spectrum internal standard method to monitor the reaction end point, and determine that the crude yield of the reaction is 38 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com