Application of synergistic aza-graphene with manganese oxide to near infrared nitrogen removal
A near-infrared light, graphene technology, applied in the direction of oxidized water/sewage treatment, chemical instruments and methods, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1)NG-α-MnO 2 Preparation: Weigh manganese chloride and potassium permanganate (the molar ratio is 3:2), and dissolve them in 20mL deionized water respectively, add 5% nitrogen-assorted graphite of manganese dioxide theoretical yield in the manganese chloride solution Alkene, sonicated for 1 hour, transferred to a three-necked flask, heated to 85°C in a water bath, and potassium permanganate was added dropwise, refluxed for 12 hours, then filtered and washed, and vacuum-dried at 70°C for 12 hours to obtain the aza Graphene-α-manganese dioxide nanocomposite photocatalyst.
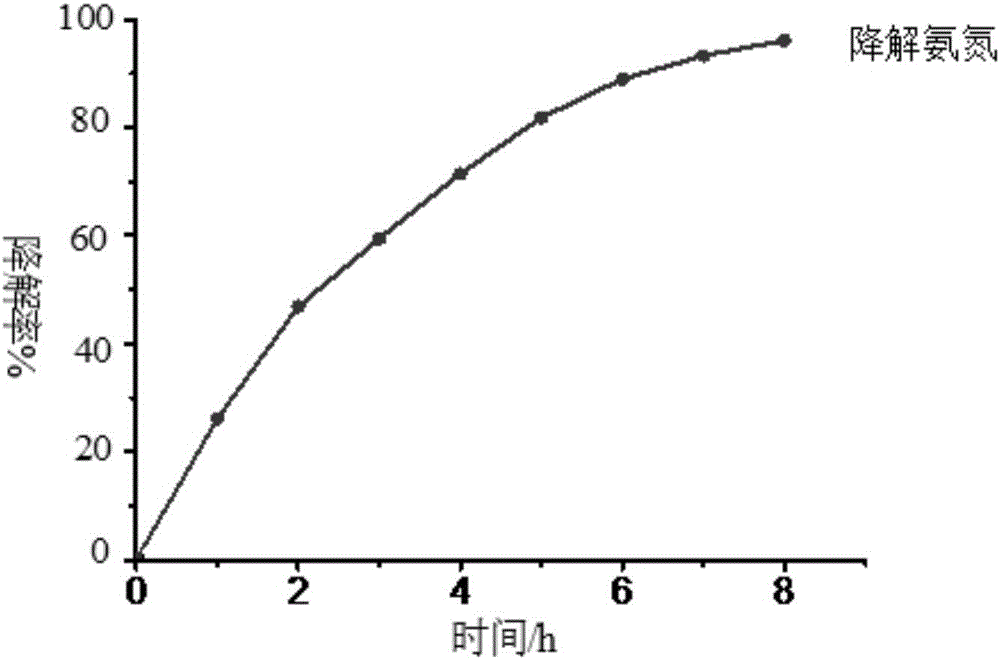
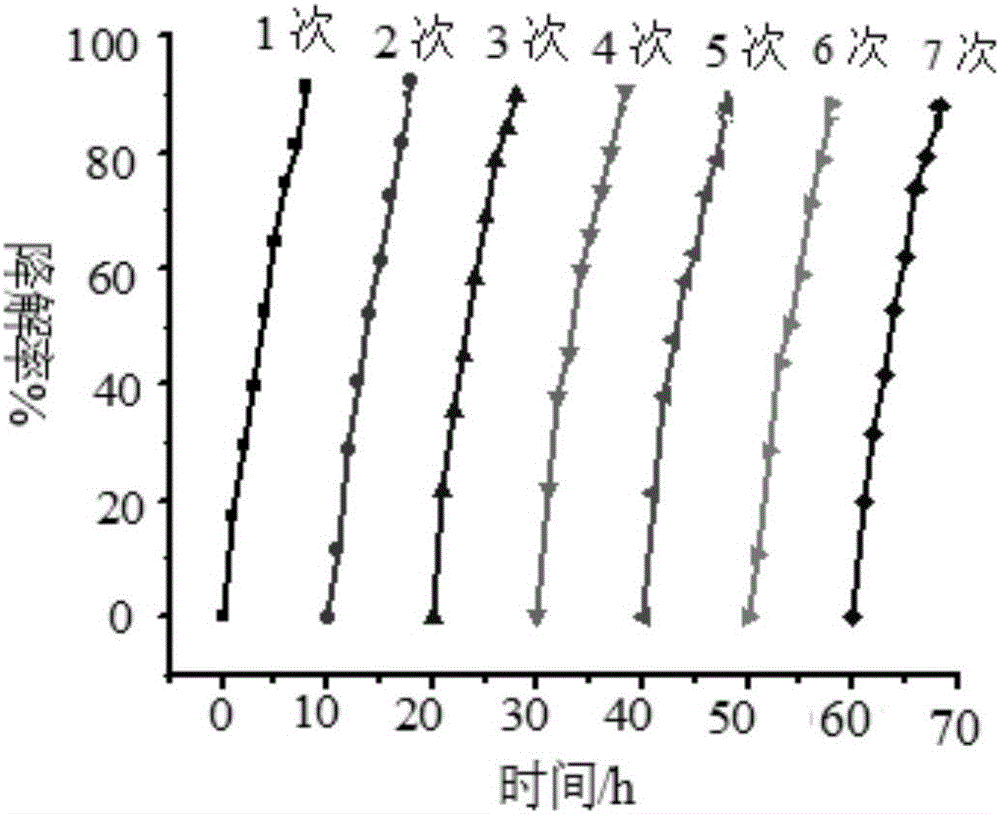
[0039] (2) Photocatalysis experiment: Wrap the wall of a 100ml beaker with tin foil to prevent ultraviolet light and visible light from entering the reaction system, and cover the mouth of the beaker with a λ>780nm cut-off filter to ensure that only near-infrared light is present. The radiation enters the photoreactor, and a 300W UV-Vis lamp is placed above the reactor. Add a certain concentration o...
Embodiment 2
[0045] NG-β-MnO 2 Preparation: Weigh manganese chloride and potassium permanganate (the molar ratio is 3:2), dissolve them in 20mL deionized water respectively, then add 5% azagraphene of manganese dioxide theoretical yield in the manganese chloride solution, Ultrasound for 1 hour, add potassium permanganate solution, stir for 30 minutes, transfer to a 100ml reaction kettle, heat at 160°C for 12h, cool to room temperature, filter and wash, and dry at 70°C for 12h under vacuum to obtain the nitrogen Heterographene-β-manganese dioxide nanohybrid photocatalyst.
[0046] (2) Photocatalysis experiment: Wrap the wall of a 100ml beaker with tin foil to prevent ultraviolet light and visible light from entering the reaction system, and cover the mouth of the beaker with a λ>780nm cut-off filter to ensure that only near-infrared light is present. The radiation enters the photoreactor, and a 300W UV-Vis lamp is placed above the reactor. Add a certain concentration of ammonia nitrogen s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com