Spiro ring-type organic materials and organic electroluminescent devices using them
An electroluminescent device and luminescent technology, applied in the direction of electric solid state devices, organic chemistry, electrical components, etc., to achieve the effects of improving machinability, increasing glass transition temperature, and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: the preparation of compound 1
[0110]
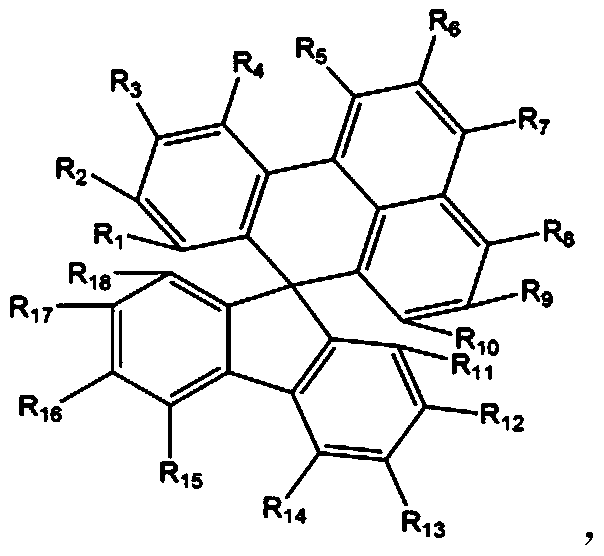
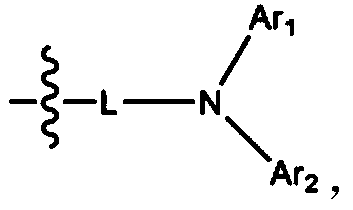
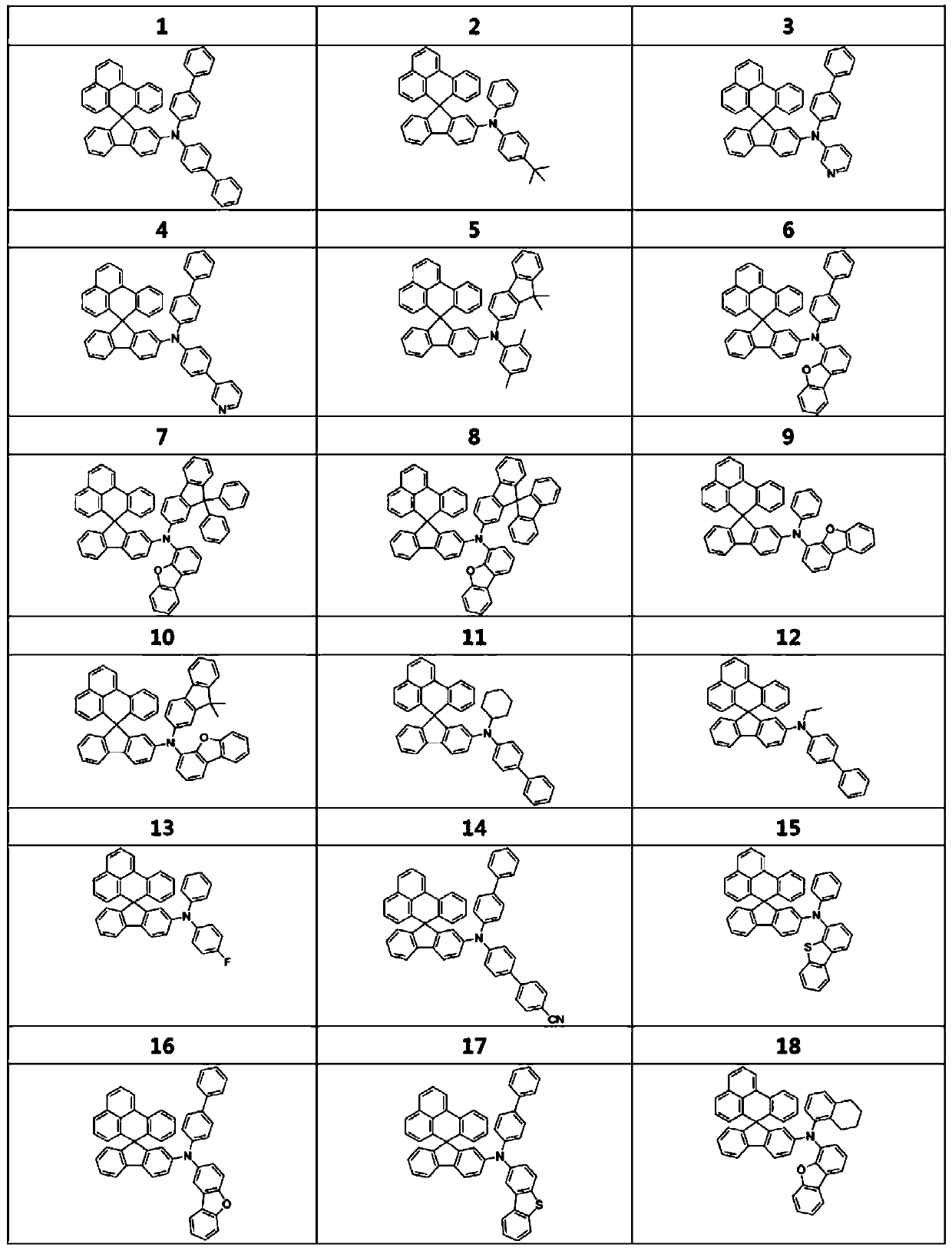
[0111] Dissolve 3.21g (10mmol) of bis([1,1'-biphenyl]-4-yl)amine and 4.45g (10mmol) of 2'-bromospiro[benzo[de]anthracene- 7,9'-fluorene], 2.88 g (30 mmol) of sodium tert-butoxide, 161 mg (0.4 mmol) of tri-tert-butylphosphine (50 wt % in toluene), 115 mg (0.2 mmol) of bis(dibenzyl Proxyl acetone) palladium (0), followed by stirring and reflux for 3 hours. After finishing the reaction, cool at room temperature, and add 50ml of ethyl acetate and 50ml of H 2 O to extract the organic layer. Using MgSO 4 After treating the organic layer, it was filtered, dried, and columnized with n-hexane / dimethylammonium chloride to obtain Compound 1 in a yield of 85% (5.83 g).
Embodiment 2
[0112] Embodiment 2: the preparation of compound 2
[0113]Instead of using 4.58 g (10 mmol) of N-phenylspiro[benzo[de]anthracene-7,9'-fluorene]-2'-amine and 2.13 g (10 mmol) of 1-bromo-4-(tert-butyl Base) benzene to replace bis([1,1'-biphenyl]-4-yl)amine and 2'-bromospiro[benzo[de]anthracene-7,9'-fluorene], in order to implement Compound 2 was prepared in the same manner as Example 1.
Embodiment 3
[0114] Embodiment 3: the preparation of compound 6
[0115] Using 3.35 g (10 mmol) of N-([1,1'-biphenyl]-4-yl)dibenzo[b,d]furan-4-amine and 4.77 g (10 mmol) of 2'-bromospiro [Benzo[de]anthracene-7,9'-fluorene] instead of bis([1,1'-biphenyl]-4-yl)amine and 2'-bromospiro[benzo[de]anthracene-7 , 9'-fluorene], Compound 6 was prepared in the same manner as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com