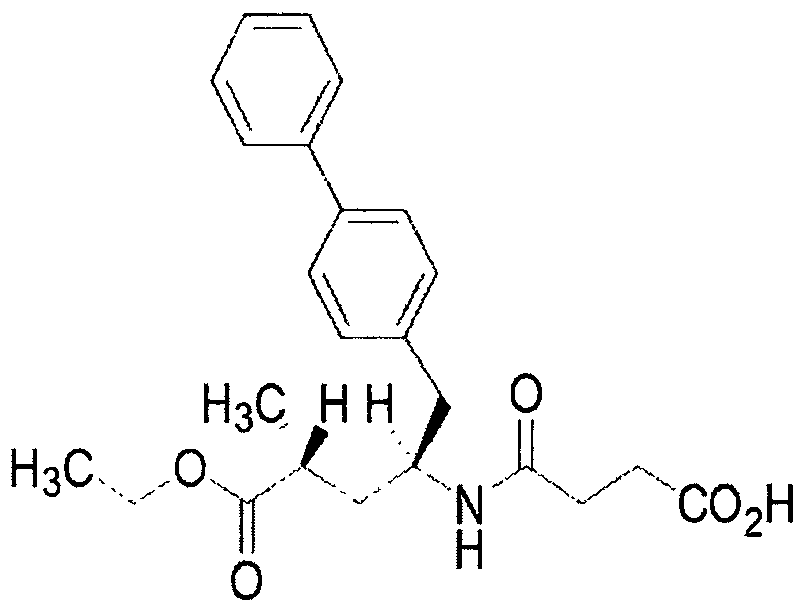
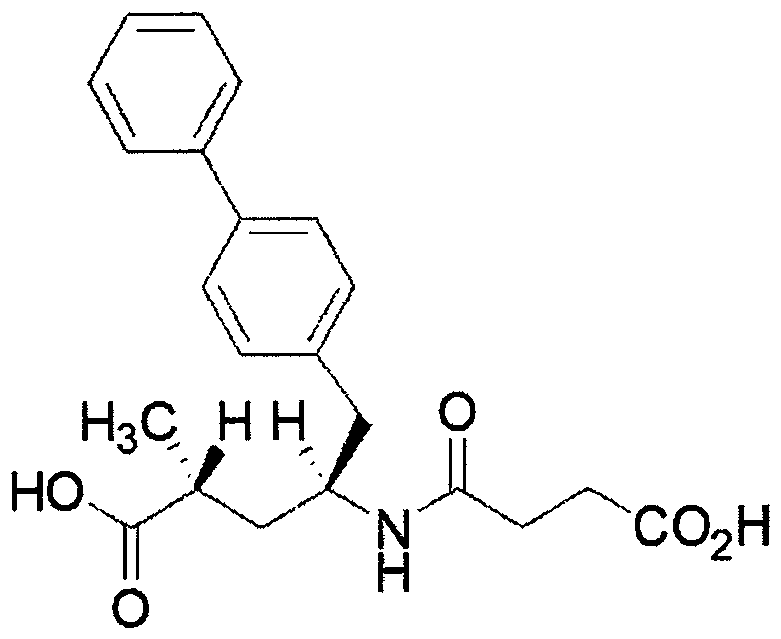
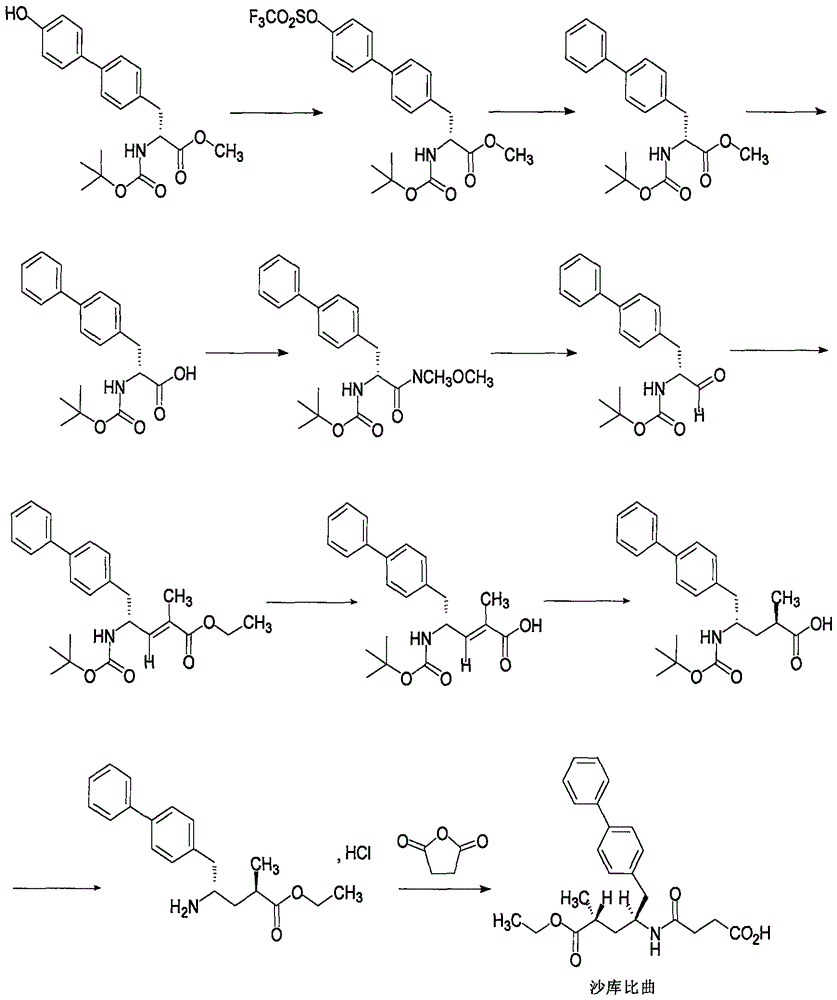
Eutectic drug for treating heart failure
A technology of co-crystals and drugs, applied in the field of medicine, can solve the problems of long synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Sacubitra Calcium Synthesis:
[0059] Control the temperature at 10-20°C, add 518g of dichloromethane, 39g of SV-A and 12.3g of succinic anhydride to a 1L dry reaction kettle in sequence, add 17.4g of N,N-diisopropylacetic acid dropwise at a temperature of 10-20°C Amine (DIPEA), after dripping, continue to control the temperature and stir for 3 to 4 hours; TLC monitors the reaction (developing solvent: ethyl acetate: acetic acid = 100: 1, Rf sv-1 = 0.2, Rf sv-2 =0.6, UV 254nm ), when SV-A disappears, the reaction is considered complete.
[0060] After the reaction is complete, add 40kg of water, evaporate the solvent under reduced pressure at 25-35°C, with a vacuum degree of ≤-0.08MPa, add 350g of isopropyl acetate to the residue, and wash the organic phase twice with water (100g / time), 0.2 % sodium bicarbonate washing 2 times (100g / time), 1.5N hydrochloric acid washing 2 times (100g / time), water washing 2 times (100g / time) and saturated sodium chloride washing 2 tim...
Embodiment 3
[0074] The HPLC detection method of Sacubi Trifemasartan Potassium:
[0075] Take Example 2 to prepare 5 mg of sacubi, trifemasartan potassium, dissolve it in a mixed solution (70 ml of mobile phase A: 30 ml of mobile phase), ultrasonicate for 10 minutes, filter, and take the filtrate.
[0076] Chromatographic column: octadecylsilane bonded silica gel as filler (recommended chromatographic column is Waters SunFire C18, 150×4.6mm, 3.5μm, or equivalent)
[0077] Mobile phase: 0.05% trifluoroacetic acid solution is mobile phase A, acetonitrile is mobile phase B, and elution is carried out according to the following gradient:
[0078] time (minutes) Mobile phase A(%) Mobile phase B(%) 0 70 30 50 35 65 50.01 70 30 60 70 30
[0079] Detection wavelength: 254nm
[0080] Flow rate: 1.0ml / min
[0081] Injection volume: 10 μl.
Embodiment 4
[0083] Sacubi Trifemasartan Potassium Tablets are formulated from the following components:
[0084]
[0085]
[0086] Its preparation method comprises the following steps:
[0087] 1) Grind and sieve sacubi, trifemasartan potassium and pharmaceutical excipients respectively, set aside,
[0088] 2) physically mix the raw materials and pharmaceutical excipients in parts by weight of the prescription amount,
[0089] 3) Wet granulation,
[0090] 4) dried, granulated,
[0091] 5) Convert the additional auxiliary materials, mix evenly, and press into tablets
[0092] 6) The Opadry is dissolved in a certain solvent and used as a coating solution for coating.
[0093] test results
[0094] Drug content: qualified
[0095] Disintegration time limit: meet the regulations
[0096] Sacubi Trifemasartan Potassium Dissolution in 45 minutes: 95.27%
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com