Pharmaceutical composition containing fimasartan and hydrochlorothiazide
A technology of Fimasartan and Hydrochlorothiazide, which is applied in the field of pharmaceutical compositions containing Fimasartan and Hydrochlorothiazide, can solve problems such as difficulty in achieving uniform mixing and preparation of granules, and achieve prevention or treatment of cardiovascular diseases and excellent physical properties , the effect of high content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
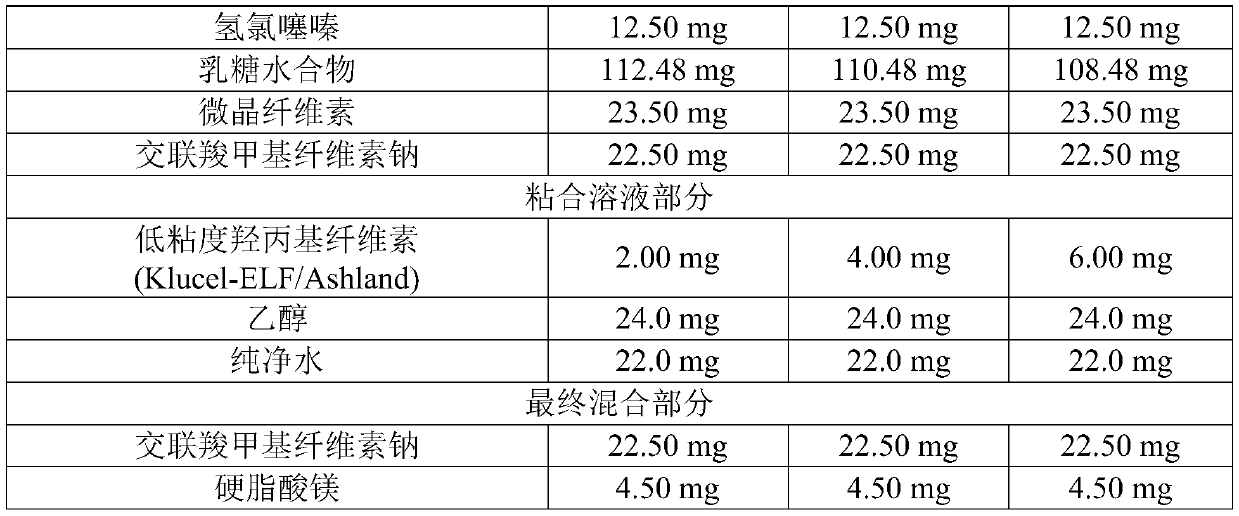
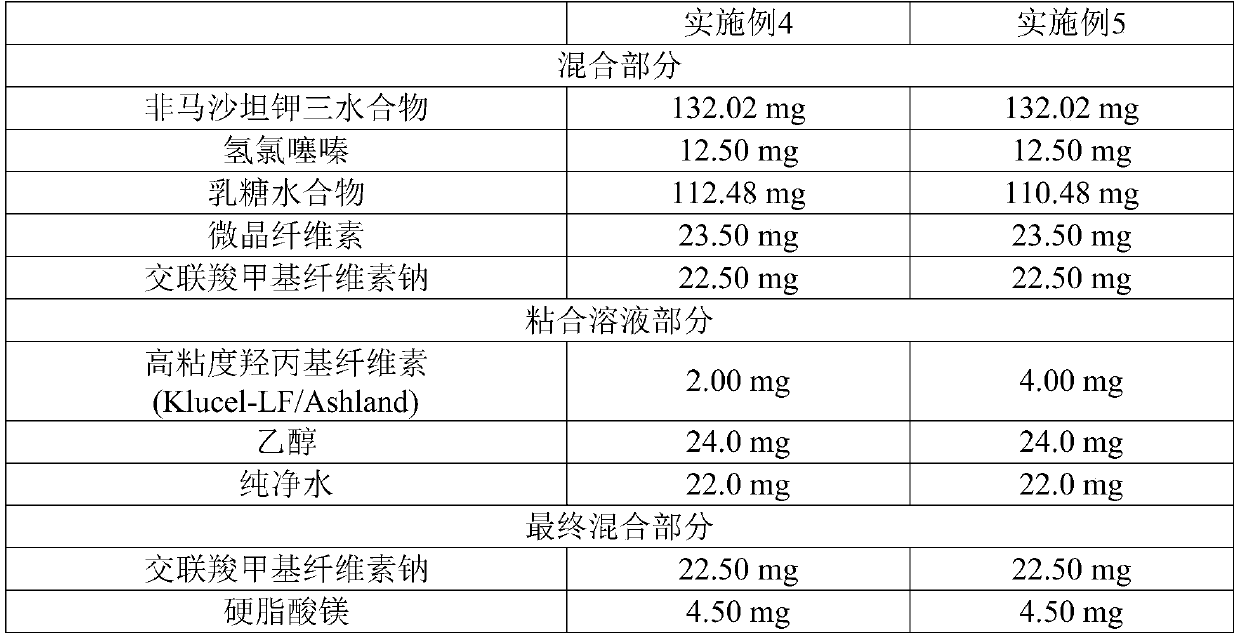
[0046] Tablets comprising Fimasartan Potassium Trihydrate and Hydrochlorothiazide were prepared using the ingredients and contents shown in Table 1 below. 132.02 mg of Fimasartan potassium trihydrate, 12.50 mg of hydrochlorothiazide, 112.48 mg of lactose hydrate, 23.50 mg of microcrystalline cellulose and 22.50 mg of croscarmellose sodium were mixed with a stirrer at 100 rpm and with a chopper (chopper) was stirred at 200 rpm for 2 minutes (high shear mixer SM-5C, Sejong Pharmatech) to prepare a mixture. A binding solution prepared by dissolving 2.00 mg of low-viscosity hydroxypropylcellulose (Klucel-ELF / Ashland) in 24.0 mg of ethanol and 22.0 mg of purified water was added to the mixture, and the mixture was stirred at 200 rpm with a cutter The grinder was stirred at 2000 rpm for 2 minutes (high shear mixer SM-5C, Sejong Pharmatech) to prepare white granules. The granules were dried at 40° C. for 10 hours, and sieved with a 30-mesh sieve to prepare a sieved material. Then, ...
Embodiment 2
[0048] A tablet comprising fimasartan potassium trihydrate and hydrochlorothiazide was prepared by substantially the same method as in Example 1, except that the ingredients and contents shown in Example 2 of Table 1 below were used.
Embodiment 3
[0050] A tablet comprising fimasartan potassium trihydrate and hydrochlorothiazide was prepared by substantially the same method as in Example 1, except that the ingredients and contents shown in Example 3 of Table 1 below were used.
[0051] [Table 1]
[0052]
[0053]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com