Sofosbuvir tablet and preparation method thereof
A technology of buvir tablet and tablet core, applied in the field of sofosbuvir tablet and its preparation, achieves the effects of reducing production cost, simple and mature production process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
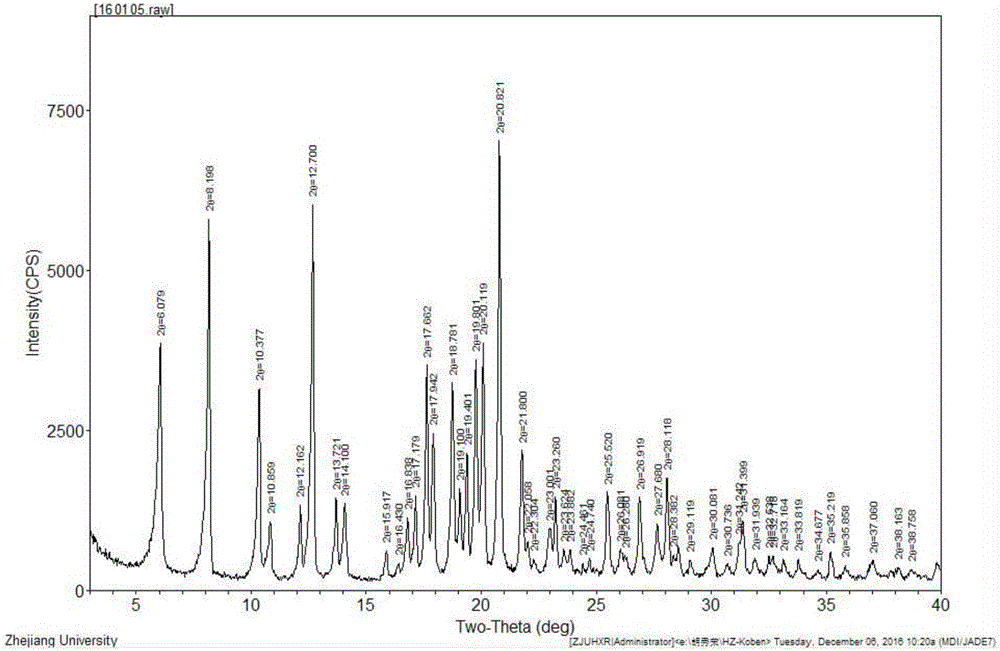
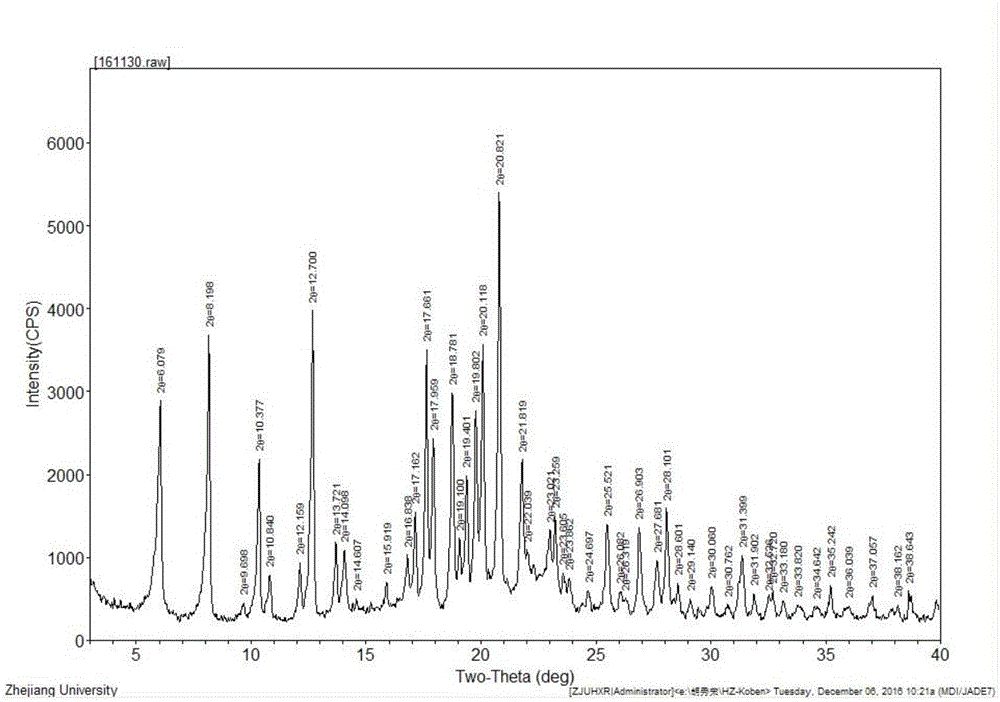
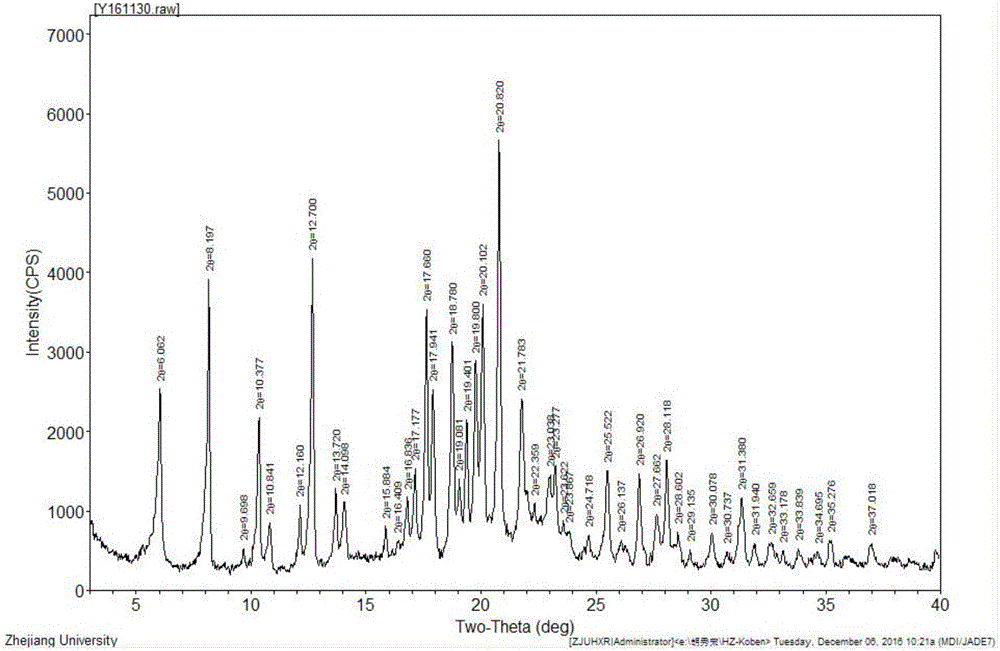
Image
Examples
Embodiment 1
[0042] Product prescription (1000 tablets):
[0043]
[0044] making process:
[0045] ① Raw material drug pretreatment: pass the sofosbuvir raw material through 80 mesh, and set aside;
[0046] ②Preparation of adhesive solution: make povidone K90 into 2% solution with water, set aside;
[0047] ③ Mixing of raw and auxiliary materials: Take the formula amount of sofosbuvir, microcrystalline cellulose, mannitol, and croscarmellose sodium, and place them in a high-shear mixing granulator and mix evenly (stirring paddle: 120rpm, 10min );
[0048]④Granulation: add 2% povidone K90 solution to granulate (spraying time: 4min; stirring speed: 150rpm; shearing speed: 1200rpm), dry (60°C, control material moisture ≤ 3.5%), granulate (40 mesh );
[0049] ⑤Total mixing: put the sorted granules and formula amount of micropowder silica gel in the mixing machine and mix evenly (speed: 15rpm, 2min);
[0050] ⑥ Tablet compression: place the granules in a high-speed rotary tablet press,...
Embodiment 2
[0053] Product prescription (1000 tablets):
[0054]
[0055]
[0056] making process:
[0057] ① Raw material pretreatment: pass the sofosbuvir raw material through 80 meshes, and set aside;
[0058] ②Preparation of adhesive solution: make povidone K30 into a 5% solution with water, and set aside;
[0059] ③ Mixing of raw and auxiliary materials: Take the formula amount of sofosbuvir, microcrystalline cellulose, pregelatinized starch, and crospovidone, and mix them evenly in a high-shear mixing granulator (stirring paddle: 120rpm, 10min) ;
[0060] ④Granulation: Add 5% povidone K30 solution to granulate (spraying time: 4min; stirring speed: 150rpm; shearing speed: 1200rpm), dry (60°C, control material moisture ≤ 3.5%), granulate (20 mesh );
[0061] ⑤Total mixing: put the sorted granules and formula amount of micropowder silica gel in the mixing machine and mix evenly (speed: 15rpm, 2min);
[0062] ⑥ Tablet compression: place the granules in a high-speed rotary tab...
Embodiment 3
[0065] Product prescription (1000 tablets):
[0066]
[0067] making process:
[0068] ① Raw material pretreatment: pass the sofosbuvir raw material through 80 meshes, and set aside;
[0069] ②Adhesive solution preparation: make povidone K30 into 8% solution with water, set aside;
[0070] ③Mixing of raw and auxiliary materials: take the formulated amount of sofosbuvir, microcrystalline cellulose, pregelatinized starch, mannitol, and 1 / 2 the formulated amount of croscarmellose sodium, place in high-shear mixing and granulate Mix evenly in the machine (stirring paddle: 120rpm, 10min);
[0071] ④Granulation: Add 5% povidone K30 solution to granulate (spraying time: 4min; stirring speed: 150rpm; shearing speed: 1200rpm), dry (60°C, control material moisture ≤ 3.5%), granulate (20 mesh );
[0072] ⑤ Blending: Put the sorted granules, the magnesium stearate of the recipe amount and 1 / 2 the croscarmellose sodium of the recipe amount in the blender and mix evenly (speed: 15rpm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com