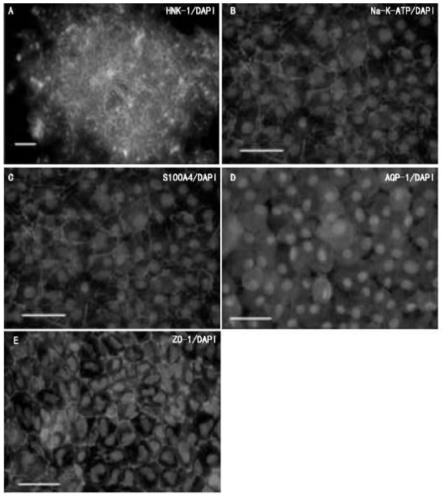
Method for obtaining corneal endothelial cells
A corneal endothelium and acquisition method technology, which is applied in the field of obtaining corneal endothelial cells, and achieves the effects of simple technical solution, broad application prospect and important clinical significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The configuration of embodiment 1 culture medium
[0034] 1. Preparation of serum-free medium
[0035] The basal medium is DMEM / F12 medium supplemented with 15% serum replacement, 15ng / mL basic fibroblast growth factor, 1× non-essential amino acid, 2mM GlutaMAX TM Medium and additives, 100 units / mL blue-chain double antibody, 0.1mM β-mercaptoethanol, prepared into a serum-free medium, stored at 4°C in the dark for future use.
[0036] 2. Preparation of differentiation medium A
[0037] The basal medium is DMEM / F12 medium supplemented with 1×N2 supplement, 12ng / mL basic fibroblast growth factor, 1×non-essential amino acid, 2mM GlutaMAX TM Culture medium and additives, 100 units / mL blue-chain double antibody, 2ng / mL recombinant human insulin, prepared as differentiation medium A, and stored at 4°C in the dark for future use.
[0038] 3. Preparation of Differentiation Medium B
[0039] The basal medium is DMEM / F12 medium supplemented with 10% serum replacement, 1× non-...
Embodiment 2
[0047] The acquisition method of embodiment 2 class corneal endothelial cells
[0048] 1. Cultivation of Commercialized Human Embryonic Stem Cells
[0049] Mouse embryonic fibroblasts were routinely cultured, and the 3-4 passages were taken as feeder layers, and the proliferation was induced in the medium supplemented with leukemia inhibitory factor (LIF), and the undifferentiated state was maintained.
[0050] 2. Acquisition of Embryoid Bodies
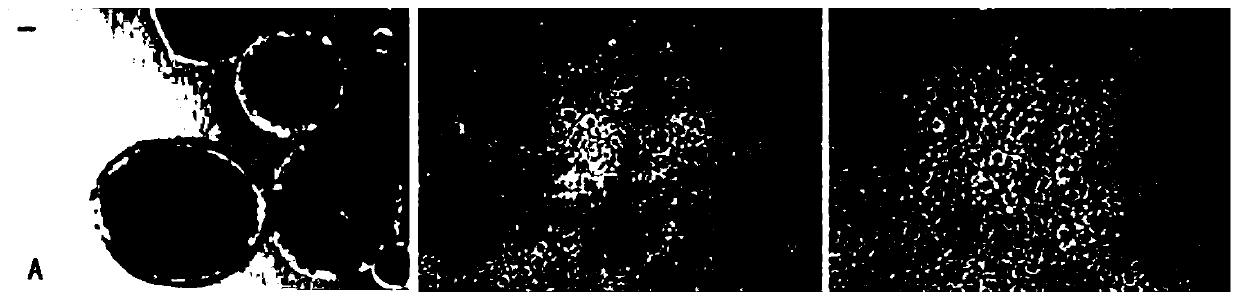
[0051] After the above-mentioned cultured commercial human embryonic stem cells were prepared and digested with collagenase IV and Dispase II at a ratio of 1:2, the prepared serum-free medium was used to culture human embryonic stem cells until they differentiated to form rosettes, that is, pseudo embryoid body, such as figure 1 as shown in a.
Embodiment 3
[0052] Embodiment 3 pre-differentiation culture
[0053] 1. Operation 1 of pre-differentiation culture
[0054] The embryoid bodies obtained in Example 2 were cultured in the differentiation medium A prepared in Example 1 supplemented with 500 ng / mL Noggin and 10 μM TGF-β1 receptor inhibitor for 4 days;
[0055] 2. Operation 2 of pre-differentiation culture
[0056] The embryoid bodies obtained in Example 2 were cultured in the differentiation medium B prepared in Example 1 supplemented with 500 ng / mL Noggin and 10 μM TGF-β1 receptor inhibitor for 2 weeks;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com