Preparation and after-treatment method for high-purity alogliptin benzoate
A technology for the preparation of benzoic acid, which is applied in the fields of metabolic diseases, organic chemistry, drug combination, etc., can solve problems such as cumbersome operation, and achieve the effects of improving yield and quality, convenient operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
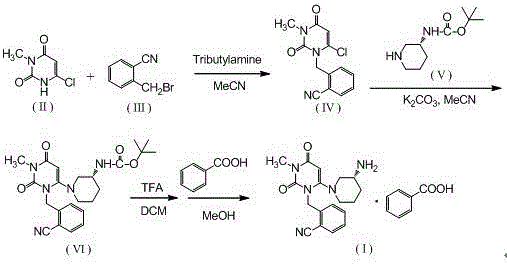
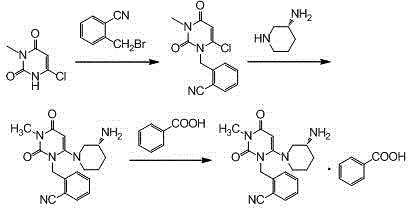
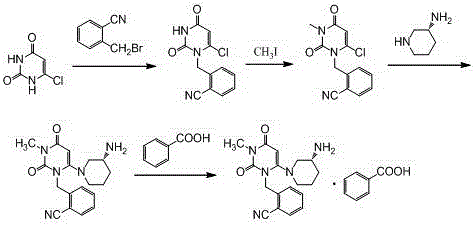
[0029] Step 1, the synthesis of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile:
[0030] Add 600ml of acetonitrile, 100.0g of o-bromomethylbenzonitrile, 81.9g of 3-methyl-6 chlorouracil, and 113.5g of tri-n-butylamine into a 1000ml three-necked flask, stir, and heat up to reflux for 6 hours (TLC monitors the reaction end point , the spots of o-bromomethylbenzonitrile disappear), cool down, remove the solvent by rotary evaporation at 40°C, add 500ml of absolute ethanol, reflux and stir to dissolve, cool down to below 10°C for crystallization, filter, and filter the cake with 100ml of cyclohexane After washing and air drying at 60°C, 121.9 g of the product was obtained with a yield of 86.7% and a purity of 98.97% by HPLC.
[0031] Synthesis of the alogliptin of step 2, Boc protection:
[0032] Add 600ml of acetonitrile, 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (intermediate Body 1) 100.0g, (R)-3-Boc-amin...
Embodiment 2
[0036] Basically the same as embodiment 1, on this basis:
[0037] Step 3: Synthesis of Alogliptin Benzoate
[0038] Add 2.8L of dichloromethane and 1410g of Boc-protected alogliptin (intermediate 2) into a 10L reactor, stir, add 2750g of trifluoroacetic acid dropwise at room temperature, and stir at room temperature for 8 hours after the addition is complete (TLC monitors the reaction end point, Intermediate 2 spots disappear). After adding 20L ethyl acetate to the reaction system and stirring evenly, wash 3 times with 2mol / L hydrochloric acid, 3L each time, combine the water phase, add 20% sodium hydroxide solution to adjust the pH of the system to 10-11, and wash with dichloromethane Extract the aqueous phase 3 times, 3 L each time, combine the organic phases, wash with 1.2 L saturated brine, add 500 g of anhydrous sodium sulfate to dry, spin the filtrate, add 8.5 L of methanol and stir to dissolve, cool down to below 5 °C, add 392 g After the benzoic acid was stirred and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com