Catalyst for catalyzing hydrogenation reduction of nitrophenol and application thereof
A technology of nitrophenol and p-nitrophenol, applied in the field of nano-catalysts, can solve the problems of reduced catalytic efficiency, poisoning and deactivation, and high cost, and achieves the effects of high catalytic activity, fast reaction speed, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
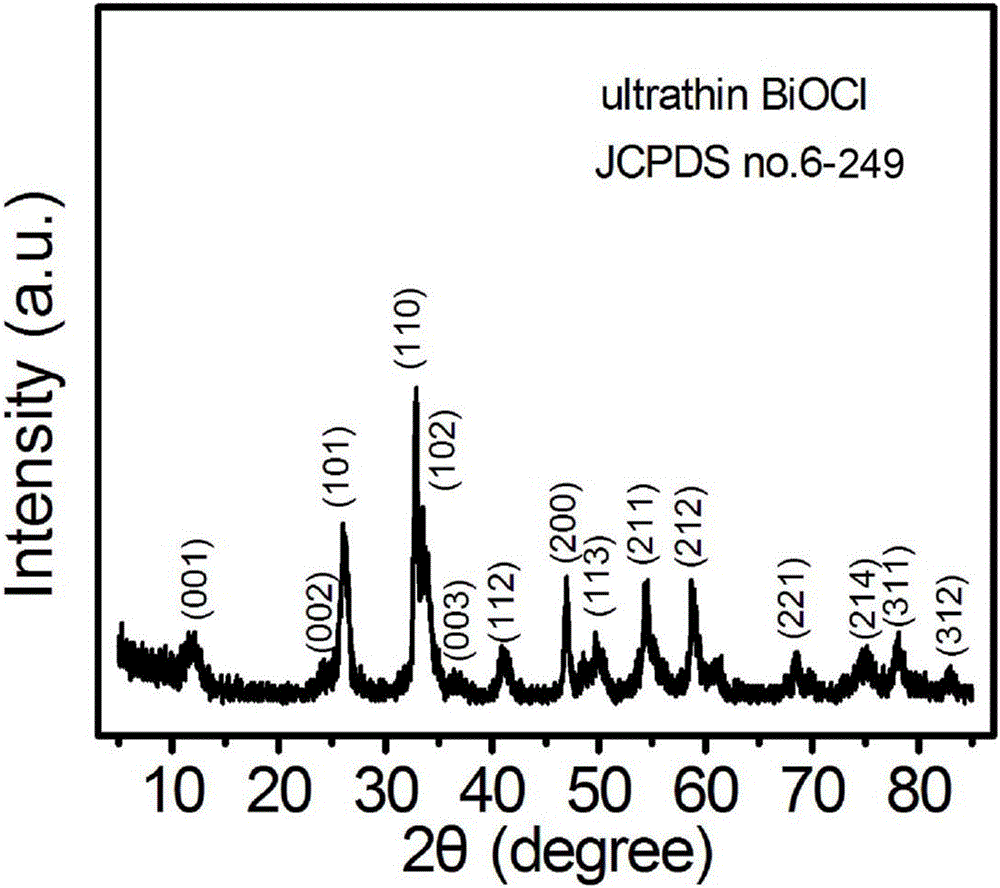
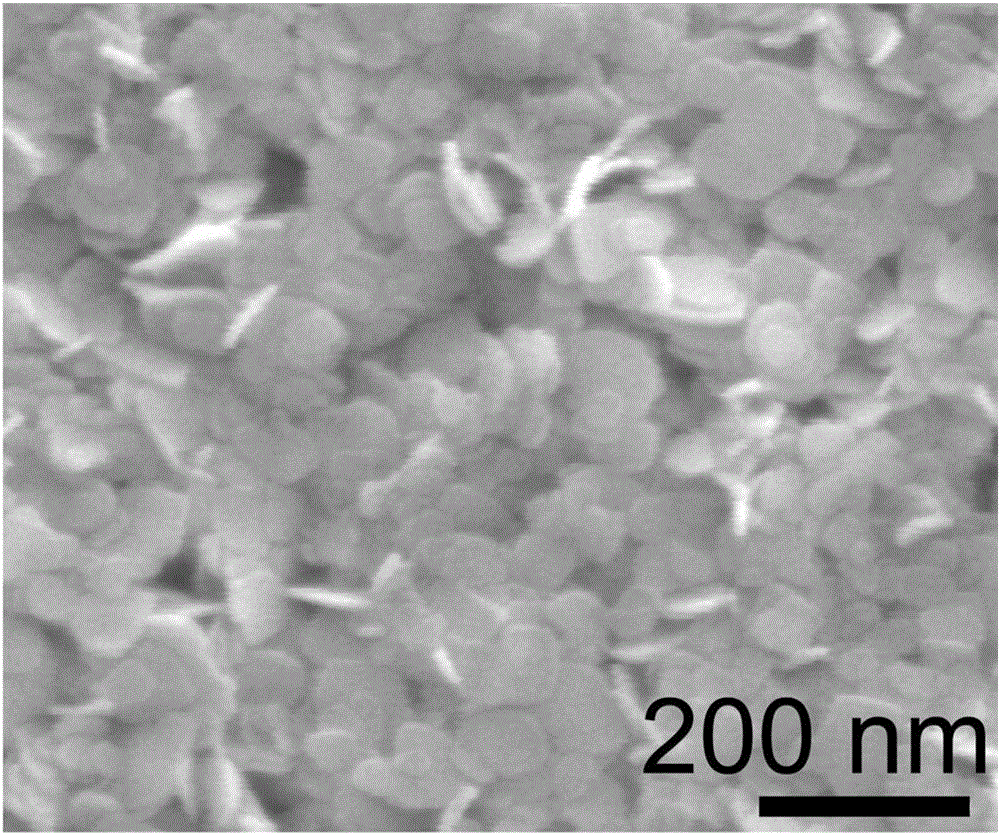
[0032] Preparation of bismuth oxychloride (BiOCl) ultrathin nanosheets:
[0033] a. Dissolve 0.6mmol mannitol and 0.4g polyvinylpyrrolidone (PVP, K-30) in 60mL distilled water, stir to dissolve;
[0034] b. Dissolve 2.0 mmol of bismuth nitrate pentahydrate in 10 mL of ethylene glycol, and ultrasonically form a solution at room temperature;
[0035] c. Dissolve 2.0mmol sodium chloride in 10mL ethylene glycol, and ultrasonically form a solution at room temperature;
[0036] d. adding the solutions prepared in step b and step c to the solution in step a successively, and stirring evenly to obtain a reaction solution;
[0037] e. Move the reaction solution in step d into a hydrothermal reaction kettle with a volume of 50mL, then seal the hydrothermal reaction kettle, keep it at 160°C for 8h, cool to room temperature after the reaction, and pass the precipitate generated in the reaction kettle through After centrifugal separation, washing with distilled water and drying, the BiOC...
Embodiment 2
[0041] Catalytic reduction of o-nitrophenol to o-aminophenol
[0042] a. Weigh 10 mg of bismuth oxychloride ultrathin nanosheets and ultrasonically disperse them in 10 mL of distilled water;
[0043] b. Pipette 0.1 mL of the suspension prepared in step a, and add it to 2.7 mL of o-nitrophenol aqueous solution (concentration: 0.1 mM);
[0044] c. Weigh 0.1134g of sodium borohydride and dissolve it in 10ml of ice water, pipette 0.4ml into the solution obtained in step b, mix quickly and evenly, and the reaction begins;
[0045] d. Use an ultraviolet-visible spectrophotometer to detect the absorption spectrum of the reaction solution in step c every 1 min, and monitor the reaction progress in real time until the peak around 400 nm no longer changes significantly.
[0046] From Figure 6 As can be seen in a, the characteristic absorption peak of o-nitrophenol itself is at 351nm, but after adding sodium borohydride, because o-nitrophenolate ions are formed under alkaline conditions...
Embodiment 3
[0048] Catalytic reduction of m-nitrophenol to m-aminophenol
[0049] a. Weigh 10 mg of bismuth oxychloride ultrathin nanosheets and ultrasonically disperse them in 10 mL of distilled water;
[0050] b. Pipette 0.1 mL of the suspension prepared in step a, and add it to 2.7 mL of m-nitrophenol aqueous solution (concentration is 0.1 mM);
[0051] c. Weigh 0.1134g of sodium borohydride and dissolve it in 10ml of ice water, pipette 0.4ml into the solution obtained in step b, mix quickly and evenly, and the reaction begins;
[0052] d. Use a UV-Vis spectrophotometer to detect the absorption spectrum of the reaction solution in step c, so that the reaction progress can be detected in real time.
[0053] Figure 7 a is the ultraviolet-visible absorption spectrogram of reaction solution in the m-nitrophenol process of embodiment 3 catalytic reduction generation m-nitrophenol, the characteristic absorption peak of m-nitrophenol itself is at 350nm place, but after adding sodium borohy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com