Application of ZNF383 protein in preparing product for inhibiting activity of p53 protein
A protein, product technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1, the construction of plasmid and the preparation of oligomeric nucleic acid
[0097] 1. Construction of Myc-ZNF383 plasmid
[0098] The small fragment between the recognition sequences of the restriction endonucleases SalI and NotI of the pCMV-Myc plasmid was replaced with the DNA molecule shown in Sequence 2 in the sequence listing, and the resulting recombinant plasmid was the Myc-ZNF383 plasmid.
[0099] The Myc-ZNF383 plasmid expresses the ZNF383 protein shown in Sequence 1 in the Sequence Listing.
[0100] 2. Construction of Flag-p53 plasmid
[0101] The small fragment between the recognition sequences of the restriction endonucleases EcoRI and BamHI of the pCMV-Flag plasmid is replaced with the DNA molecule shown in sequence 4 in the sequence table, and the resulting recombinant plasmid is the Flag-p53 plasmid.
[0102] The Flag-p53 plasmid expresses the p53 protein shown in sequence 3 in the sequence listing.
[0103] 3. Preparation of oligonucleo...
Embodiment 2
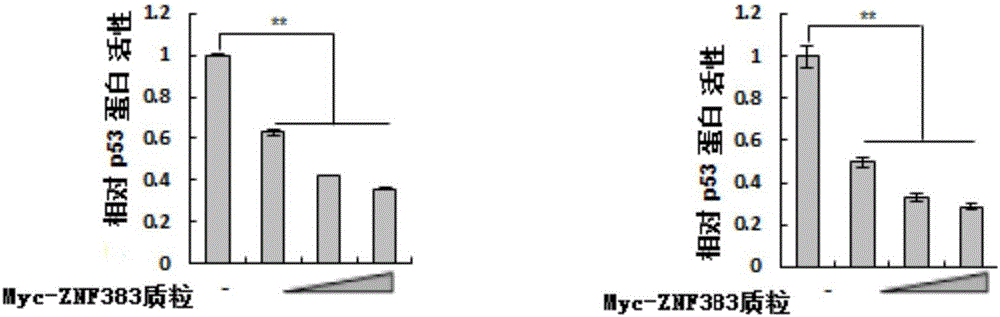
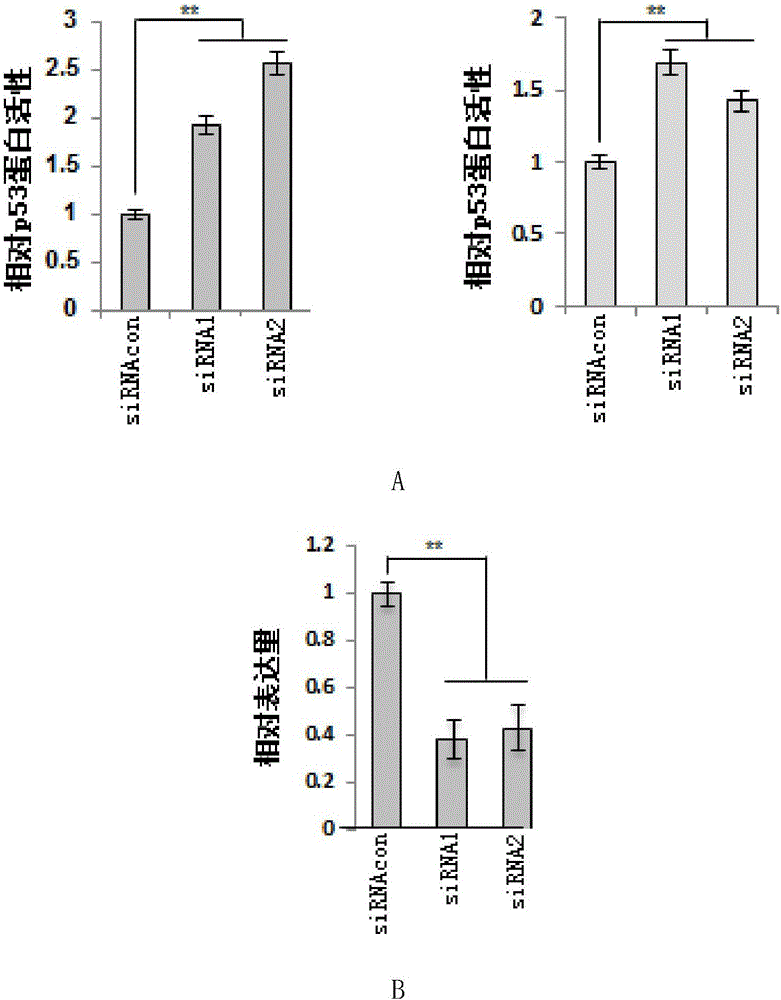
[0110] Example 2, ZNF383 protein inhibits the activity of endogenous p53 protein in a dose-dependent manner
[0111] 1. Experiment 1
[0112] The experiment was repeated three times to take the average value, and the steps for each repetition were as follows:
[0113] 1. Cells (p53 + / + HCT116 cells or Hela cells) were seeded in 24-well plates containing 0.5 mL DMEM medium (8.0×10 per well 4 cells), and then placed at 37°C, 5% CO 2 Cultivate in an incubator, and when the fusion rate reaches 70-90%, they are randomly divided into four groups, and each group is set with three replicate wells, and the following treatments are carried out:
[0114] The first group: 20ng pG13L plasmid, 0.2ng pRL-TK plasmid and 0.4μg pCMV-Myc plasmid were added to each well for co-transfection;
[0115] The second group: add 0.1μg Myc-ZNF383 plasmid, 20ng pG13L plasmid, 0.2ng pRL-TK plasmid and 0.3μg pCMV-Myc plasmid to each well for co-transfection;
[0116] The third group: 0.2 μg Myc-ZNF383 p...
Embodiment 3
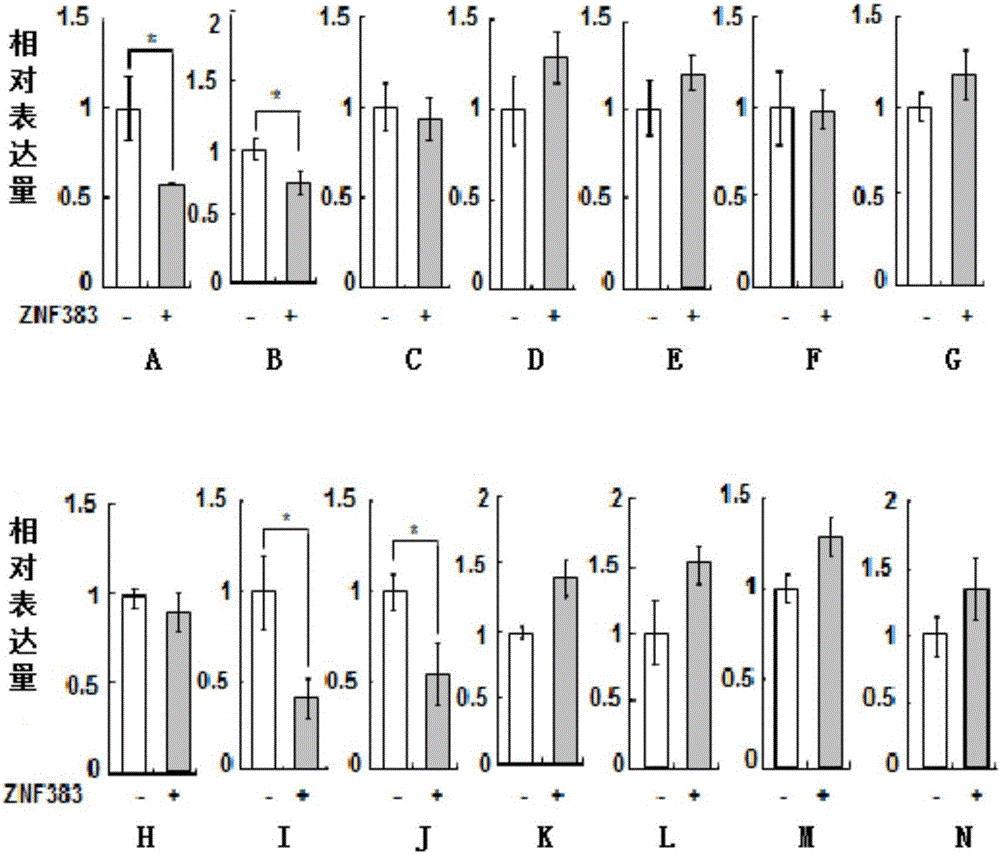
[0134] Example 3. ZNF383 protein depends on p53 protein to selectively down-regulate the expression of downstream apoptosis and immune-related target genes of p53 gene
[0135] 1. Experiment 1
[0136] The experiment was repeated three times to take the average value, and the steps for each repetition were as follows:
[0137] 1. Convert p53 + / + HCT116 cells were seeded in 6-well plates containing 2 mL of DMEM medium (2.0 × 10 per well 5cells), and then placed at 37°C, 5% CO 2 Culture in an incubator, and when the fusion rate reaches 70-90%, they are randomly divided into two groups, and each group is set with three replicate wells, and the following treatments are carried out:
[0138] Group 1: Add 2.0 μg pCMV-Myc plasmid to each well for co-transfection;
[0139] The second group: 2.0 μg Myc-ZNF383 plasmid was added to each well for co-transfection.
[0140] 2. 48 hours after step 1 was completed, the total RNA of each cell was extracted respectively, and then the first...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com