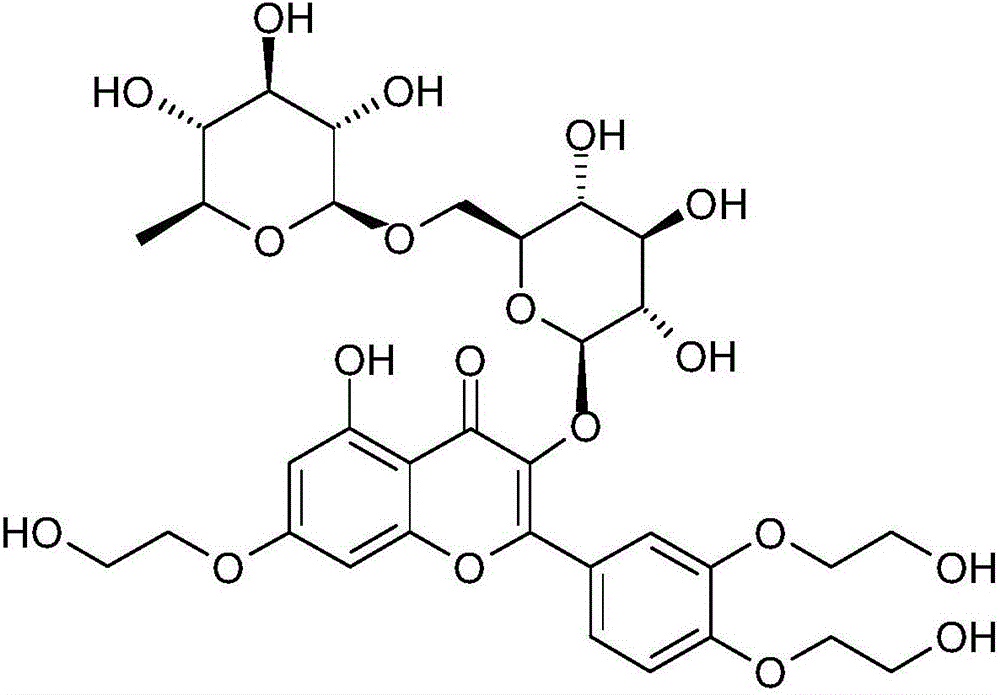
Preparing method of 3',4',7'-troxerutin
A technology of trihydroxyethyl rutin and rutin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as methanol waste, reduce waste generation, simple production process, and reduce waste. The effect of reducing the by-products of butyoxyhydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under room temperature, 200kg of reaction solvent water is pumped into the reaction tank, then 61kg of rutin is dropped into, and the catalyst potassium hydroxide solution (containing potassium hydroxide 8.14kg) is added dropwise under the nitrogen protection stirring condition, and then chloroethanol 24.4kg is slowly added under the liquid surface. kg, heated up to 65°C, reacted at constant temperature for 4 hours, then added potassium hydroxide solution (containing potassium hydroxide 4.1kg), continued to react for 16 hours, then took samples, and after the reaction was completed by HPLC, cooled to 18°C in an ice-water bath, nitrogen The pH was adjusted to 4.5 with hydrochloric acid under protection. Distill the reaction liquid to dryness under reduced pressure, add 6 times of methanol with the solid mass obtained by distillation under reduced pressure, stir and heat to reflux at 65°C for half an hour, cool down to 20°C and crystallize for 10 hours, centrifuge and fi...
Embodiment 2
[0037] Under room temperature, 2000kg of reaction solvent water is pumped in the reaction tank, then drop into rutin 610kg, drip catalyst potassium hydroxide solution (containing potassium hydroxide 82kg) under nitrogen protection stirring condition, then slowly add chloroethanol 244kg under the liquid level, Raise the temperature to 67°C, react at constant temperature for 4.5 hours, then add potassium hydroxide solution (containing potassium hydroxide 40kg), continue to react for 18 hours, then take samples, after the HPLC detection is complete, cool to 20°C in an ice-water bath, adjust with hydrochloric acid under nitrogen protection pH to 5.2. Distill the reaction liquid to dryness under reduced pressure, add 5 times of the solid mass of methanol obtained by distillation under reduced pressure, stir, heat to 68°C and reflux for 40 minutes, cool down to 22°C and crystallize for 11 hours, and centrifuge to obtain 3',4',7-trihydroxy Ethyl rutin crude product, the obtained moth...
Embodiment 3
[0039]Under room temperature, 2000kg of reaction solvent water is pumped in the reaction tank, then drop into rutin 610kg, drip catalyst potassium hydroxide solution (containing potassium hydroxide 79kg) under the nitrogen protection stirring condition, then slowly add chloroethanol 246kg under the liquid level, Raise the temperature to 68°C, react at constant temperature for 4 hours, then add potassium hydroxide solution (containing potassium hydroxide 42kg), continue to react for 20 hours, then take samples, after the HPLC detection is complete, cool to 18°C in an ice-water bath, adjust with hydrochloric acid under nitrogen protection pH to 6. Distill the reaction liquid to dryness under reduced pressure, add 4 times of methanol with the solid mass obtained by distillation under reduced pressure, stir and heat to 75°C for reflux for 45 minutes, cool down to 24°C for crystallization for 12 hours, and centrifuge to obtain 3',4',7-trihydroxy Ethyl rutin crude product, the obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com