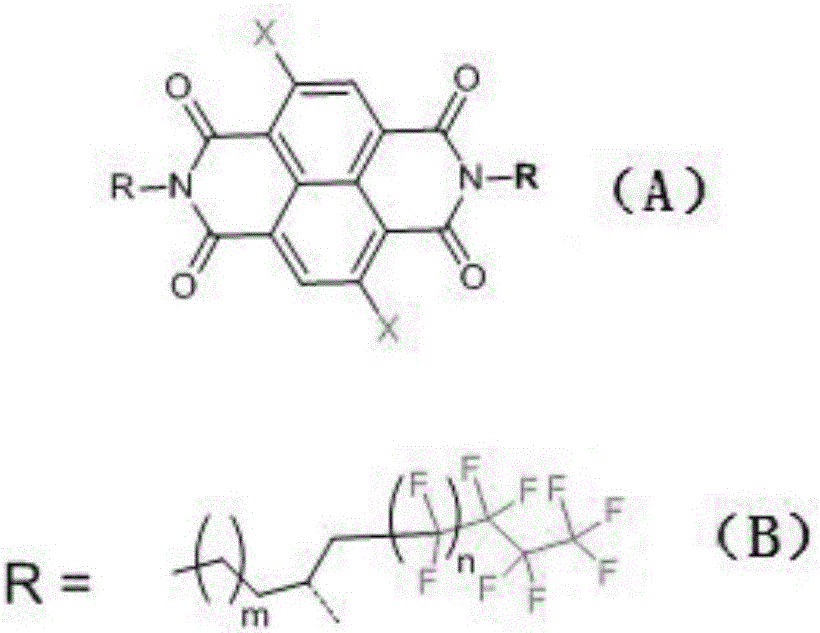
Perfluoroalkyl group modified solution-processable naphthalimide and preparation method thereof
A technology of perfluoroalkylamine and perfluoroalkyl, which is used in naphthalene dicarboxamide dyes/phthalimide dyes, organic chemistry, etc., can solve the problems of unsuitable solution processing and poor solubility, and achieve enhanced photothermal Stability, the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
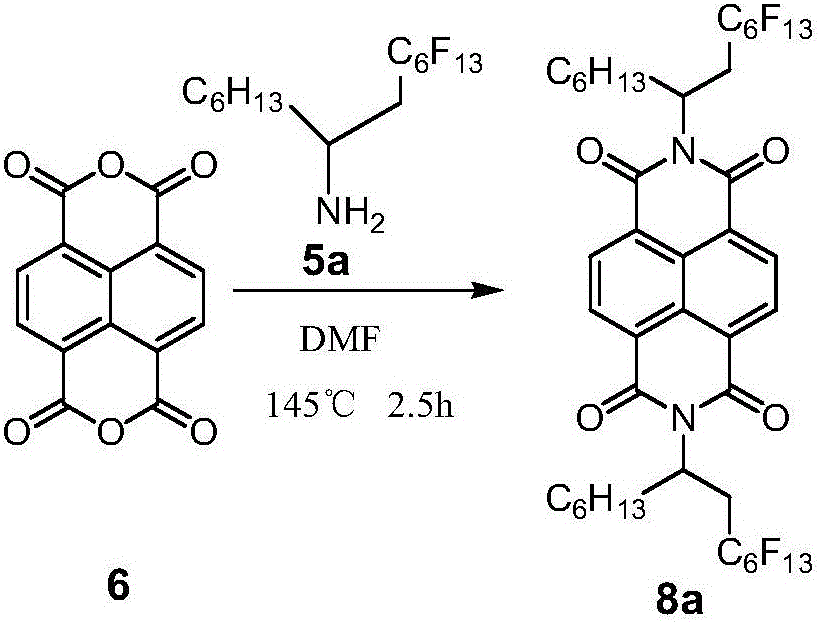
[0015] Example 1 N, N'-bis(2-perfluorohexyl-1-hexylethyl)naphthalimide (8a)
[0016]
[0017] Add compound 6 (0.268g, 1mmol), compound 5a (1.342g, 3mmol), DMF (15mL) in a 25mL two-necked flask, react at 145°C for 2.5h, cool to room temperature, neutralize with 2mol / L HCl, and CH 2 Cl 2 Extracted, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, rotary evaporated, dichloromethane and petroleum ether column separated to obtain white solid 8a (0.411g, 36.5%). 1 HNMR (400MHz, CDCl 3 )δ H :8.72-8.80(d,4H),5.68(m,2H),3.27-3.37(m,2H),2.21-2.57(m,4H),1.90-2.01(d,2H),1.21-1.31(m, 16H),0.82-0.84(m,6H);HRMS(MALDI-TOF):Calcd for C 42 h 36 f 26 N 2 o 4 1126.2260,found:1126.2278(M - ).
Embodiment 2
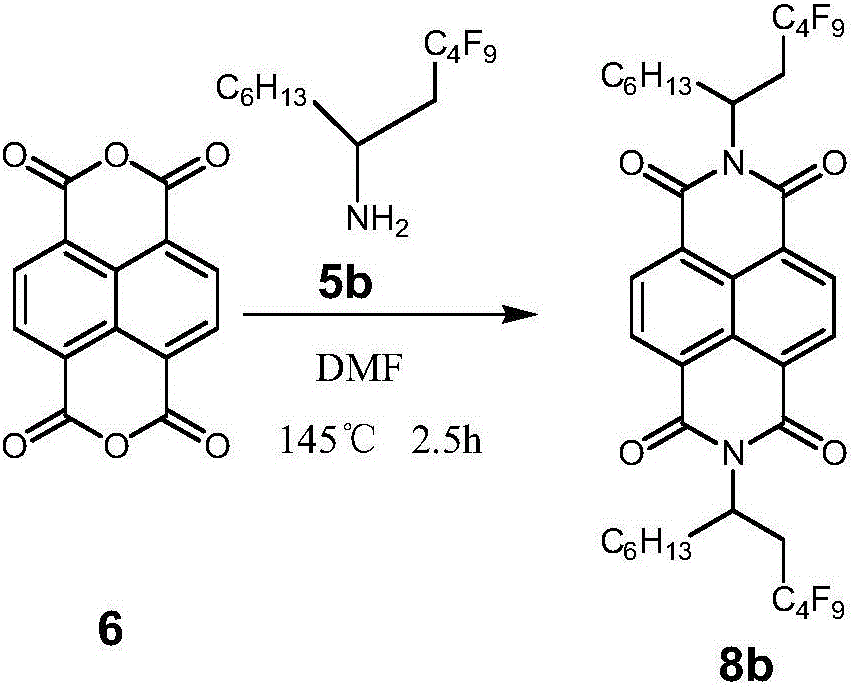
[0018] Example 2 N, N'-bis(2-perfluorobutyl-1-hexylethyl)naphthalimide (8b)
[0019]
[0020] Add compound 6 (0.801g, 3mmol), compound 5b (3.125g, 9mmol), DMF (30mL) in a 100mL two-necked flask, react at 145°C for 2.5h, cool to room temperature, neutralize with 2mol / L HCl, and use CH 2 Cl 2 Extracted, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, rotary evaporated, dichloromethane and petroleum ether column separated to obtain white solid 8b (1.281g, 40%). 1 HNMR (400MHz, CDCl 3 )δ H :8.73-8.80(d,4H),5.68(m,2H),3.29-3.37(m,2H),2.21-2.58(m,2H),1.91-2.01(d,2H),1.21-1.34(m, 16H),0.81-0.84(m,6H);HRMS(MALDI-TOF):Calcd for C 38 h 36 f 18 N 2 o 4 926.2388, found: 926.2396 (M - ).
Embodiment 3
[0021] Example 3 N, N'-bis(2-perfluorohexyl-1-butylethyl)naphthalimide (8c)
[0022]
[0023] Add compound 6 (0.536g, 2.102mmol), compound 5c (2.515g, 8.405mmol), DMF (15mL) in a 25mL two-necked flask, react at 130°C for 2.5h, cool to room temperature, neutralize with 2mol / L HCl, use CH 2 Cl 2 Extracted, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, rotary evaporated, dichloromethane and petroleum ether column separated to obtain white solid 8c (1.608g, 75%). 1 HNMR (400MHz, CDCl 3 )δ H :8.73-8.80(d,4H),5.67(m,2H),3.29-3.37(m,2H),2.21-2.58(m,4H),1.91-2.01(d,2H),1.21-1.34(m, 8H),0.82-0.85(m,6H);HRMS(MALDI-TOF):Calcd for C 38 h 28 f 26 N 2 o 4 1070.1634,found:1070.1656(M - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com