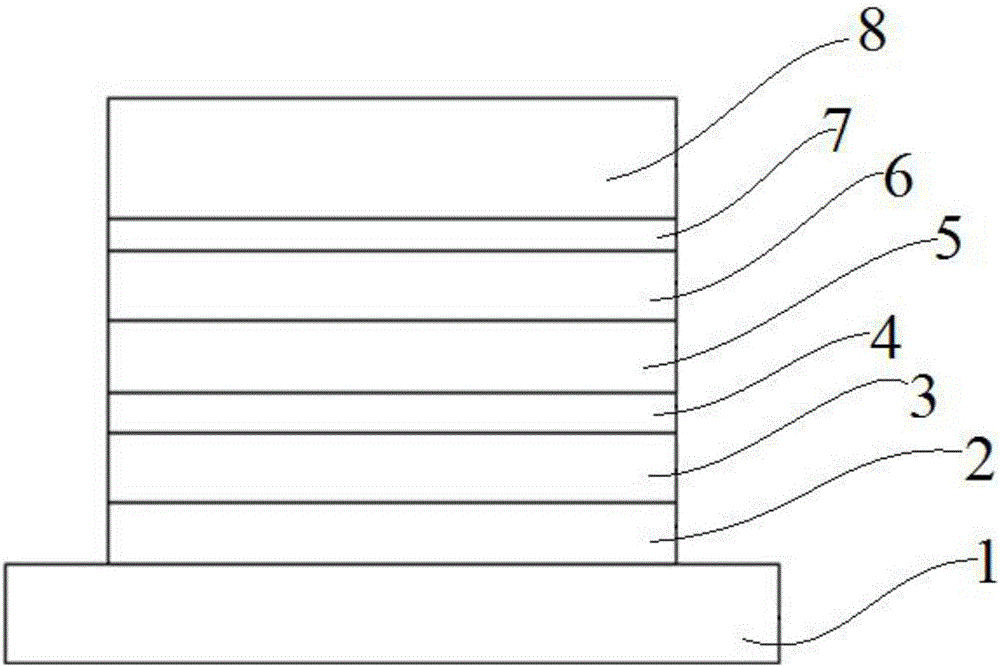
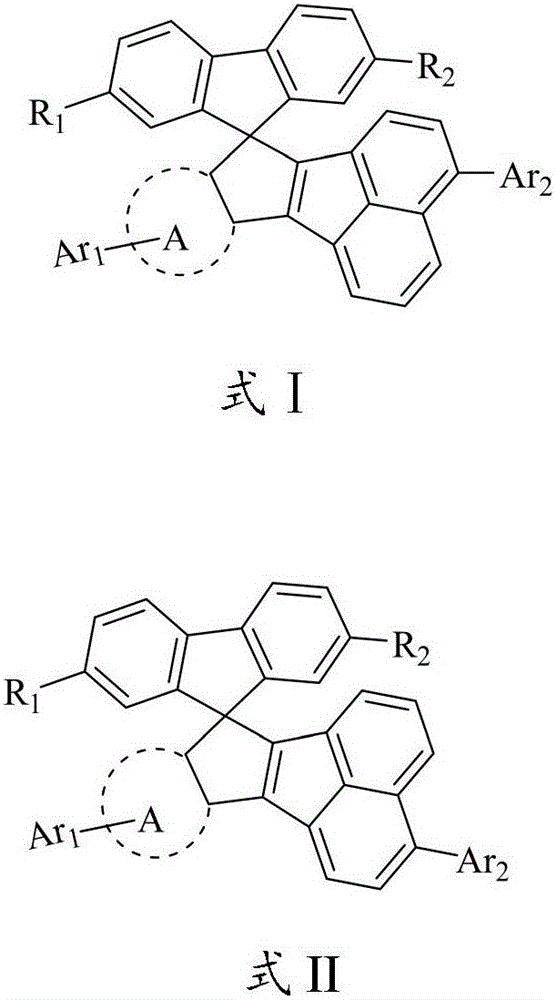
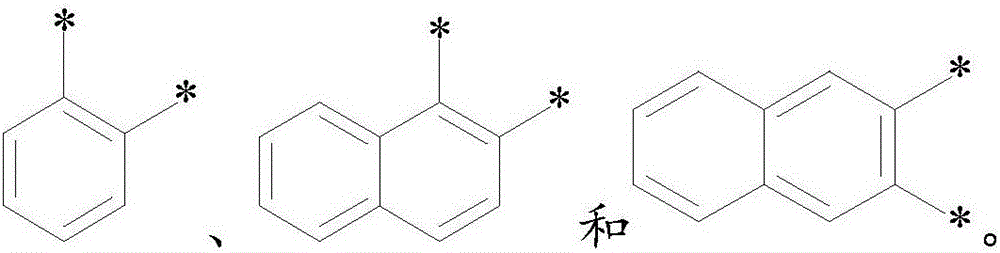
Spiro ring organic photoelectric material, preparation method and applications thereof
A technology of organic photoelectric materials and spiro rings, which is applied in the preparation of organic compounds, luminescent materials, and hydrocarbon production from halogen-containing organic compounds, etc., can solve different problems and achieve avoidance of compact accumulation, high thermal stability, and high glass transition The effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1. Preparation of organic optoelectronic materials
Embodiment 1
[0105] Example 1 Preparation of aforementioned compound 1
[0106] (1) Preparation of compound 1-a:
[0107]
[0108] At -15°C and under the protection of nitrogen, 23.1g of 2-bromo-4-hydroxybenzoic acid methyl ester (100.0mmol) and 121.4g (120mmol) of triethylamine were dispersed in 200g of dichloroethane, then drop Add 31.0 g of trifluoromethanesulfonic anhydride (110 mmol), after the dropwise addition, keep warm for 2.0 hours, then slowly warm up the reaction system to room temperature, then pour the above system into 200 g of water, stir and react for 30 min, the system is layered, and water is used to carry out the reaction. After washing, the organic phase was finally desolvated under reduced pressure until there was no fraction, and 36.1 g of compound 1-a was obtained without further purification.
[0109] (2) Preparation of compound 1-b:
[0110]
[0111] Get the 36.1g compound 1-a (100.0mmol), 20.7g potassium carbonate (150mmol), 80g water, 11.0g phenylboron...
Embodiment 2
[0133] Example 2 Preparation of aforementioned compound 8
[0134] (1) Preparation of compound 8-a:
[0135]
[0136] Take the 36.1g compound 1-a (100.0mmol), 20.7g potassium carbonate (150mmol), 80g water, 22.3g phenylboronic acid (90mmol) and 300g toluene obtained in step (1), under the protection of nitrogen, add catalyst 0.9 g Pd(PPh 3 ) 4 (0.75mmol), then heated to reflux, while adopting thin-layer chromatography (TLC) to track the reaction process, after about 6.0 hours of reaction, after being down to room temperature, the system was layered, and then after washing with water, the organic phase was decompressed and decompressed. Solvent until there is no fraction, and then use petroleum ether ethyl acetate mixture to carry out column chromatography purification to the residue, wherein the volume ratio of petroleum ether and ethyl acetate is petroleum ether:ethyl acetate=25:1, to obtain 22.5g of compound 8- a, The yield is 59.84%.
[0137] (2) Preparation of com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com