Synthesizing method for whitening agent raw materials
A synthesis method and whitening agent technology, applied in the field of synthesis of vitamin C ethyl ether, can solve the problems of no significant improvement in yield and purity, and achieve the effects of easy-to-obtain raw materials, simple process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
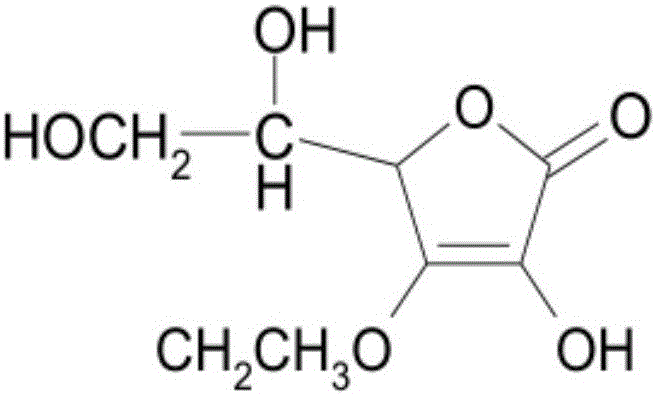
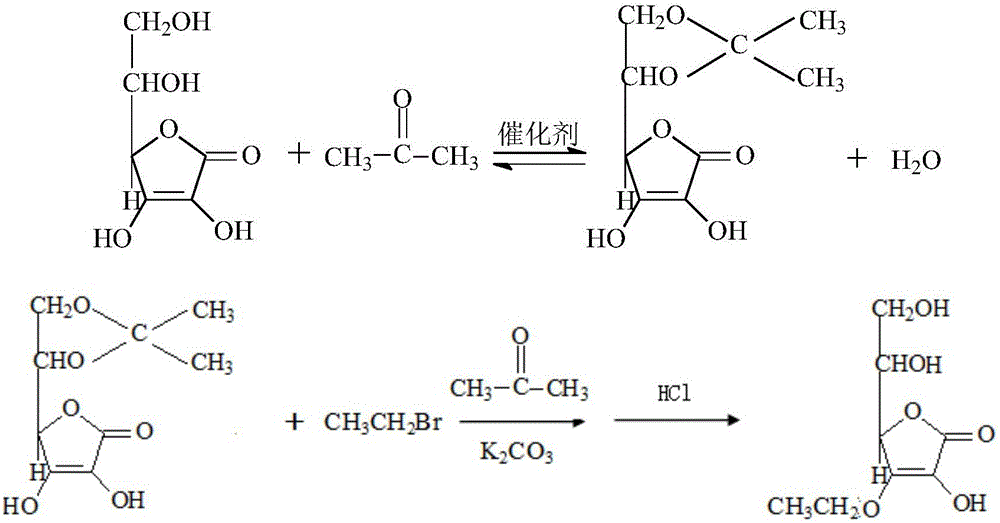
[0045] Add 0.1mol ascorbic acid (17.6g), 2mol acetone (116.2g), 0.006mol anhydrous copper sulfate (0.96g) to a 1L reactor successively, stir, react in a water bath at 30°C for 15h, and analyze by TLC during the reaction The chromatographic board is used for monitoring. After the reaction of ascorbic acid is completed, the obtained reaction solution is suction-filtered while it is hot, and the copper sulfate with crystal water is filtered out to obtain an acetone solution with 5,6-O-isopropylidene-L-ascorbic acid.
[0046] Because anhydrous copper sulfate is used as the catalyst, it will not cause the reaction to obtain a relatively acidic solution, so directly add 0.1mol (13.8g) potassium carbonate to the above solution, protect it with nitrogen, and react at about 60°C for 0.5h, then directly Add 0.1mol (10.9g) bromoethane, continue to react for 1-2h, insoluble potassium bromide is formed, after filtering, 3-O-ethane-5,6-O-isopropylidene-L- Acetone solution of ascorbic acid. ...
Embodiment 2
[0050] Add 0.1mol ascorbic acid (17.6g), 2mol acetone (116.2g), 0.006mol anhydrous copper sulfate (0.96g) to a 1L reactor successively, stir, react in a water bath at 30°C for 15h, and analyze by TLC during the reaction The chromatographic board is used for monitoring. After the reaction of ascorbic acid is completed, the obtained reaction solution is suction-filtered while it is hot, and the copper sulfate with crystal water is filtered out to obtain an acetone solution with 5,6-O-isopropylidene-L-ascorbic acid.
[0051] Because anhydrous copper sulfate is used as the catalyst, it will not cause the reaction to obtain a relatively acidic solution, so directly add 0.1mol (13.8g) potassium carbonate to the above solution, protect it with nitrogen, and react at about 60°C for 0.5h, then directly Add 0.1mol (10.9g) bromoethane, continue to react for 1-2h, insoluble potassium bromide is formed, after filtering, 3-O-ethane-5,6-O-isopropylidene-L- Acetone solution of ascorbic acid. ...
Embodiment 3
[0055] Add 0.1mol ascorbic acid (17.6g), 2mol acetone (116.2g), 0.006mol anhydrous copper sulfate (0.96g) to a 1L reactor successively, stir, react in a water bath at 30°C for 15h, and analyze by TLC during the reaction The chromatographic board is used for monitoring. After the reaction of ascorbic acid is completed, the obtained reaction solution is suction-filtered while it is hot, and the copper sulfate with crystal water is filtered out to obtain an acetone solution with 5,6-O-isopropylidene-L-ascorbic acid.
[0056] Because anhydrous copper sulfate is used as the catalyst, it will not cause the reaction to obtain a relatively acidic solution, so directly add 0.1mol (13.8g) potassium carbonate to the above solution, protect it with nitrogen, and react at about 60°C for 0.5h, then directly Add 0.1mol (10.9g) bromoethane, continue to react for 1-2h, insoluble potassium bromide is formed, after filtering, 3-O-ethane-5,6-O-isopropylidene-L- Acetone solution of ascorbic acid. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com