Preparation method for intermediates of new medicine Lusutrombopag resisting to thrombopenia
An anti-platelet, intermediate technology, applied in the field of pharmaceutical intermediates, can solve the problems of poor control, uneconomical, unenvironmental protection, etc., and achieves the effects of simple operation, easy operation and easy repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
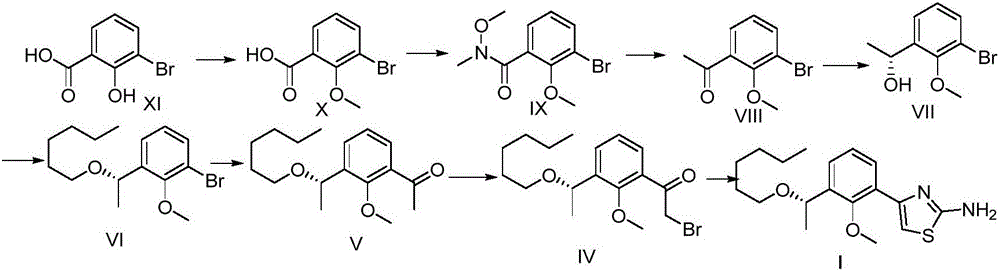
[0043] The synthetic route of intermediate I is as follows:
[0044]
[0045] 1: Synthesis of Compound X
[0046]Compound XI (360g, 1.65mol) was added to the reaction kettle, acetone (1.8L) was added to dissolve, anhydrous potassium carbonate (552g, 4.0mol) was added, and dimethyl sulfate (443g, 3.50mol) was slowly added dropwise. After the addition was complete, reflux to TLC showed that the starting material disappeared. Filter, wash the solid with acetone, combine the organic phases, concentrate, dilute with ethyl acetate, wash with water, wash with saturated brine, and dry over anhydrous sodium sulfate. After filtration and concentration, the desired compound X (355 g, 94%) was obtained, which was directly put into the next reaction.
[0047] 2: Synthesis of compound IX
[0048] Compound X (355 g, 1.55 mol) was added to the reaction kettle, dissolved in toluene, and thionyl chloride was added, and concentrated under reflux for 2 hours to obtain a yellow oily liquid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com