Lithium-rich manganese-based positive electrode material and preparation method thereof
A lithium-rich manganese-based, positive electrode material technology, applied in the direction of battery electrodes, electrical components, electrochemical generators, etc., can solve the surface structure damage of lithium-rich manganese-based positive electrode materials, can not meet the needs of electrode material performance, structural stability Reduce and other problems, to achieve the effect of reducing the first irreversible capacity loss, improving the diffusion of lithium ions, and improving the bonding strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
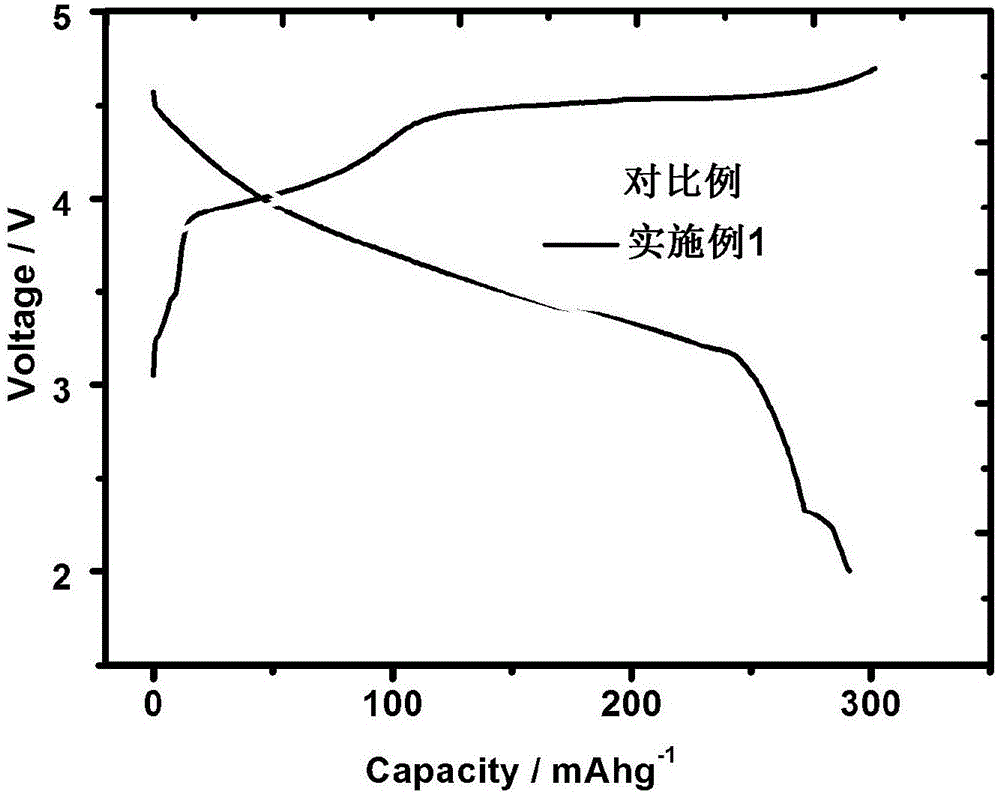
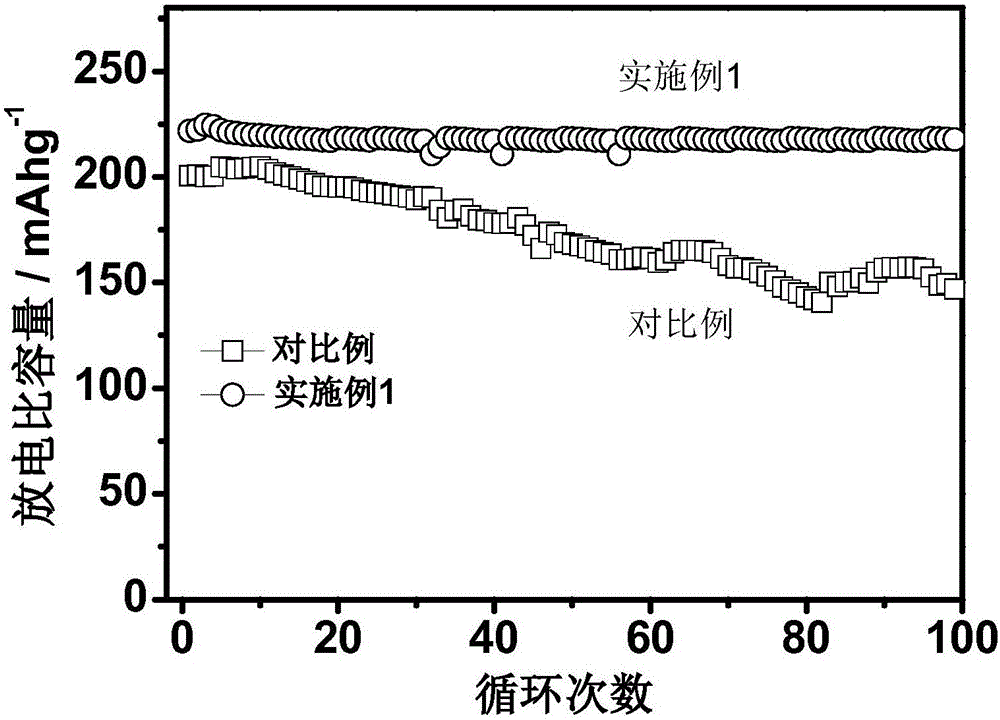
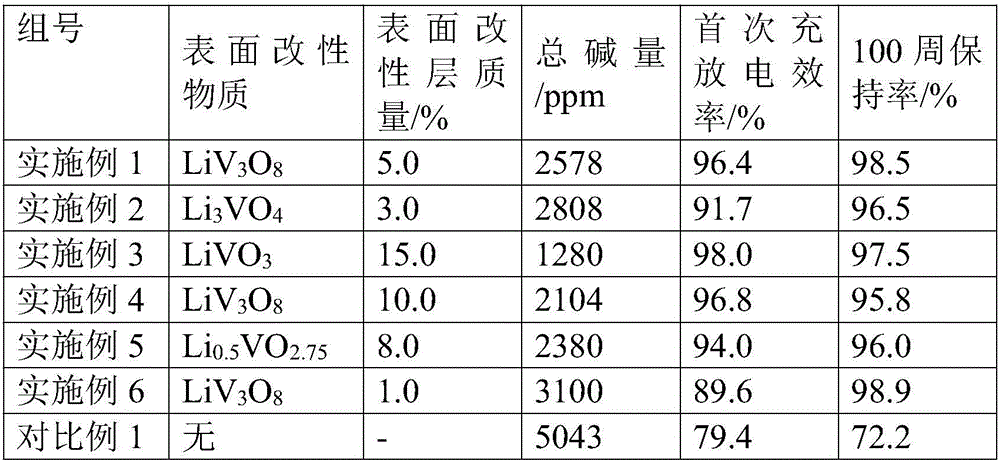
[0035] In the lithium-rich manganese base with an average particle size of 10 μm (molecular formula: Li 1.2 mn 0.56 Ni 0.16 co 0.08 o 2 ) 5.0% (mass fraction) of LiV formed on the surface of the inner core 3 o 8 (1 / 2Li 2 O 3 / 2V 2 o 5 ) surface modification layer.
[0036] Its preparation method is as follows:
[0037] Weigh 100.0g lithium-rich manganese-based core material (Li 1.2 mn 0.56 Ni 0.16 co 0.08 o 2 ), 6.098g ammonium vanadate (NH 4 VO 3 ) and 0.640g lithium carbonate (Li 2 CO 3 ) was mixed uniformly by mechanical ball milling, and then treated in air at 500°C for 4 hours to obtain LiV 3 o 8 The surface-modified lithium-rich manganese-based positive electrode material, the total alkali content of the tested modified material is 2578ppm.
[0038] The electrochemical performance test is as follows:
[0039] Mix the target product with the conductive agent acetylene black and the binder PVDF (polyvinylidene fluoride) according to the mass ratio of 8:...
Embodiment 2
[0042] In the lithium-rich manganese base with an average particle size of 5 μm (molecular formula: Li 1.2 mn 0.56 Ni 0.13 co 0.13 o 2 ) 3.0% (mass fraction) of Li formed on the surface of the inner core 3 VO 4 (3 / 2Li 2 O˙1 / 2V 2 o 5 ) surface modification layer.
[0043] Its preparation method is as follows:
[0044] Weigh 100.0g lithium-rich manganese-based cathode material (Li 1.2 mn 0.56 Ni 0.13 co 0.13 o 2 ), 2.012g vanadium pentoxide V 2 o 5 and 2.784g of lithium hydroxide (LiOH) are uniformly mixed with a mechanical fusion machine, and then treated at 800°C in the air for 10 hours to obtain Li 3 VO 4 The surface-modified lithium-rich manganese-based positive electrode material, the total alkali content of the tested modified material is 2808ppm.
[0045] Electrochemical performance test is the same as embodiment 1;
[0046] Electrochemical tests show that the first discharge specific capacity is 310.8 and 285.2mAh / g in the voltage range of 0.1C and 2.0...
Embodiment 3
[0048] In the lithium-rich manganese base material with an average particle size of 15 μm (molecular formula: Li 1.167 mn 0.533 Ni 0.2 co 0.1 o 2 ) LiVO with 15.0% (mass fraction) formed on the surface of the inner core 3 (1 / 2Li 2 O˙1 / 2V 2 o 5 ) surface modification layer.
[0049] Its preparation method is as follows:
[0050] Weigh 100.0g lithium-rich manganese-based cathode material (Li 1.167 mn 0.533 Ni 0.2 co 0.1 o 2 ), 11.754g vanadium dioxide VO 2 and 5.94g of lithium hydroxide (LiOH) are uniformly mixed by atomic layer deposition technology, and then treated in air at 900°C for 1.0h to obtain LiVO 3 The surface-modified lithium-rich manganese-based positive electrode material, the total alkali content of the tested modified material is 1280ppm.
[0051] Electrochemical performance test is the same as embodiment 1;
[0052] Electrochemical tests show that the first discharge specific capacity is 298.6 and 292.2mAh / g in the voltage range of 0.1C and 2.0-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com