Stable isotope mercapto compound labeling reagent and its synthesis method and application
A technology of thiol compounds and stable isotopes, applied in organic chemistry methods, organic chemistry, instruments, etc., can solve the problems of poor labeling accuracy and sensitivity, difficult access to labeling reagents, and weak signals of labeled products, achieving high sensitivity and selection The effect of high accuracy, fast labeling reaction rate, enhanced accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
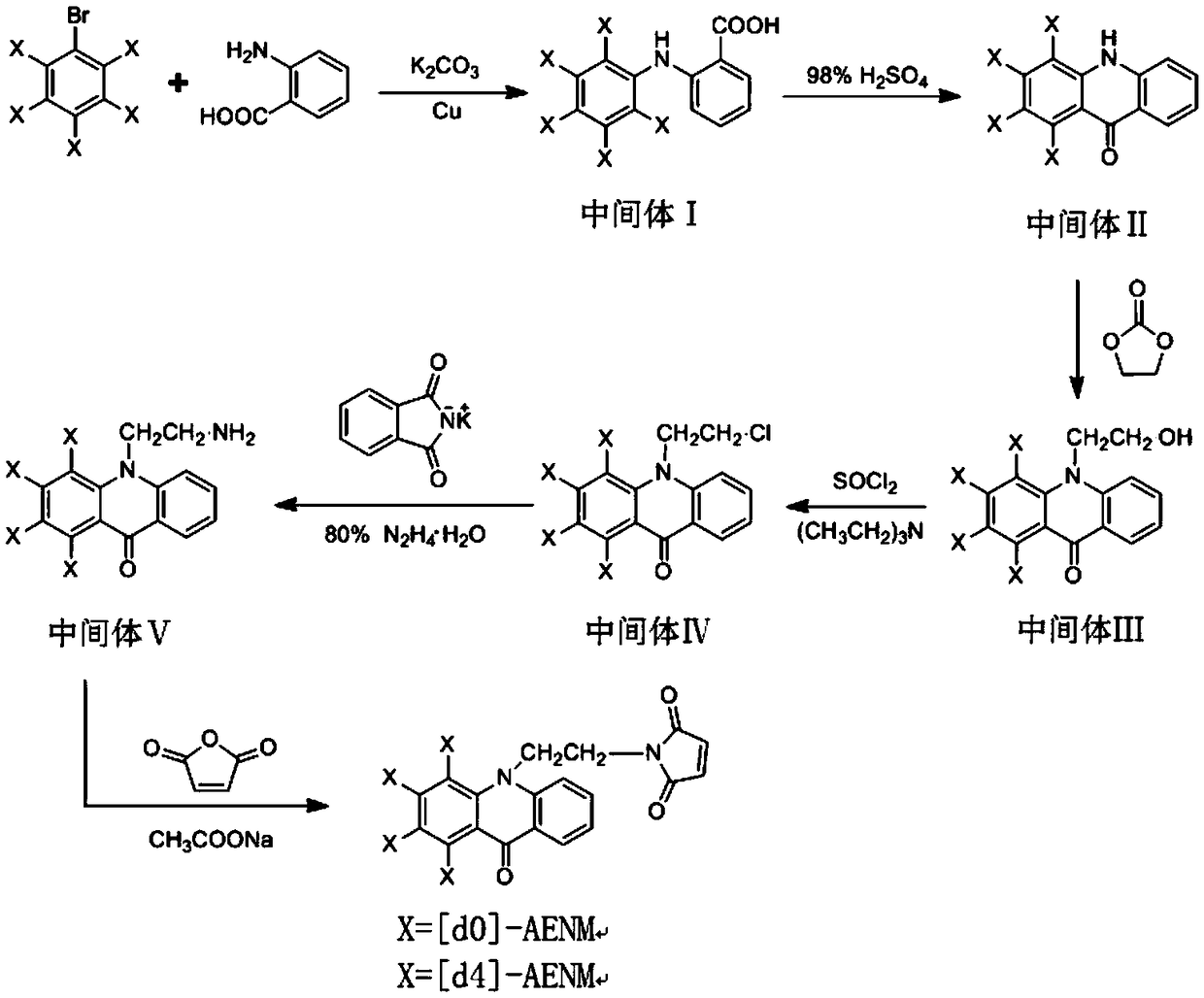
[0034] Such as figure 1 As shown, the synthesis of the stable isotope mercapto compound labeling of the present invention has 6 steps in total, and the synthetic route is as follows figure 1 , the heavy-duty labeling reagent uses [d5]-bromobenzene as a raw material, and the synthesis operation of the heavy-duty labeling reagent is as follows:
[0035] 1. Preparation of intermediate Ⅰ[d5]-2-anilinobenzoic acid
[0036] Add 22g of anthranilic acid, 20g of [d5]-bromobenzene, 10g of potassium carbonate and 0.5g of copper powder into a 250mL three-neck flask, add 150mL of nitrobenzene as a solvent, heat and reflux on a heating mantle, and stir for 3 hours; the reaction is complete Afterwards, the solvent was distilled off with steam, and the mixed reaction solution was poured into 200 ml of water, neutralized with concentrated hydrochloric acid to a pH value of 5-6, stirred thoroughly, and the precipitated solid was filtered and dried to obtain intermediate I as a gray powder . ...
Embodiment 2
[0056] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 0.01mol / L; prepare a concentration of 1×10 -4 Acetonitrile solution of [d0]-AENM and [d4]-AENM in mol / L; the preparation concentration is 1×10 -6 A series of thiol standards in mol / L (3-mercapto-hexanol, hexanethiol, furfuryl mercaptan, 2-methyl-3-furyl mercaptan, phenethanethiol, 3-mercapto-acetic acid-hexane ester, 4-methyl-4-mercapto-2-pentanone) in acetonitrile.
[0057] Add 50 μL of PBS buffer solution, 100 μL of thiol standard solution and 50 μL of acetonitrile solution of light-duty labeling reagent [d0]-AENM to a 2 mL ampoule in sequence, and react in a water bath at 40°C for 10 minutes. At the same time, under the same conditions, the real sample of wine after column purification was labeled with the heavy reagent [d4]-AENM. After the reaction was completed, they were mixed at a volume ratio of 1:1, and then analyzed by HPLC-MS / MS.
[0058] The peak area of the light derivative obtained ...
Embodiment 3
[0061] Quantification of mercaptans was performed using Agilent 1290 ultra-high performance liquid chromatography, 6460 triple quadrupole tandem mass spectrometer, equipped with electrospray ionization source (ESI). Chromatographic conditions: mobile phase A is 5% acetonitrile + 95% water, mobile phase B is 100% acetonitrile, and 0.1% formic acid is added to the mobile phase to enhance the ionization efficiency of the analyte; use gradient elution, set the mobile phase B. The initial gradient is 30%, which becomes 100% after 10 minutes and is maintained for 5 minutes; the injection volume is 1 μL, and the flow rate is 0.2 mL / min. Mass spectrometry conditions: ESI positive ion mode, drying gas temperature 300°C, flow rate 10L / min, atomization pressure 40psi, sheath gas temperature 250°C, flow rate 8L / min, capillary voltage 3.5KV. Collision voltage (Fragmentor) and fragmentation energy (CE) parameters are as follows:
[0062]
[0063] Derivatives all show strong [M+H] + Sig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com