Mutant of phenylalanine deaminase with anabaena source
A technology of phenylalanine deaminase and mutants, applied in the field of protein engineering, can solve the problems of enzyme application limitations and achieve good pH stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
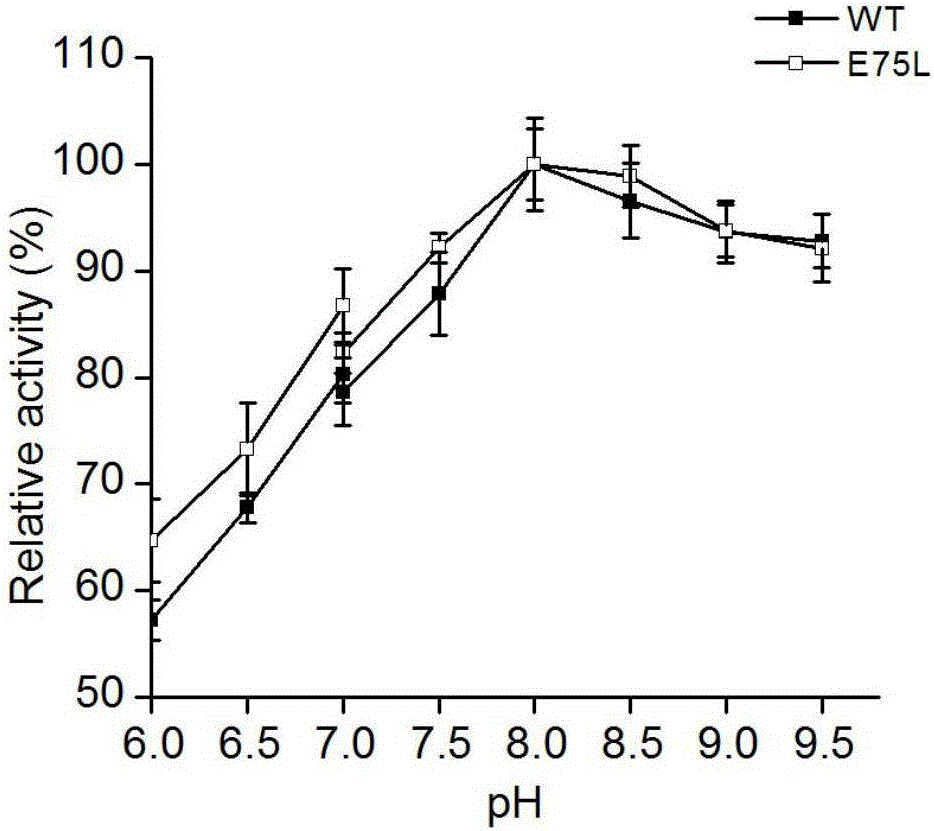
[0019] (1) Construction of mutant E75L:
[0020] Using pET28a-PAL as a template, using the primers shown in Table 1 and the conditions shown in Table 2, the recombinant vector pET28a-PAL / E75L carrying the gene encoding the mutant was obtained by PCR. For the construction method of pET28a-PAL, see Moffitt, M.C., Louie, G.V., Bowman, M.E., Pence, J., Noel, J.P. and Moore, B.S. (2007) Discovery of twocyanobacterial phenylalanine ammonia lyases: Kinetic and structural characterization. Biochemistry-Us, 46 ,1004-1012.
[0021] Table 1 Primers
[0022]
[0023] Table 2 Whole plasmid PCR amplification reaction system
[0024]
[0025]
[0026] The PCR amplification reaction conditions are:
[0027]
[0028] PCR products were identified by agarose gel electrophoresis. Then the PCR product was purified and digested and transferred to BL21 host.
[0029] (2) Inoculate recombinant Escherichia coli BL21 / pET28a-PAL / E75L in 4 mL of LB medium (peptone 10 g / L, yeast extract 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com