Sturgeon-source aeromonas hydrophila and application thereof
A technology of Aeromonas hydrophila and sturgeon, applied in the direction of bacteria, antibacterial drugs, bacterial antigen components, etc., can solve the problem of unsatisfactory immune prevention effect of heterogeneous serotype strains, and achieve the prevention of bacterial sepsis in sturgeon , the effect of good marketing prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
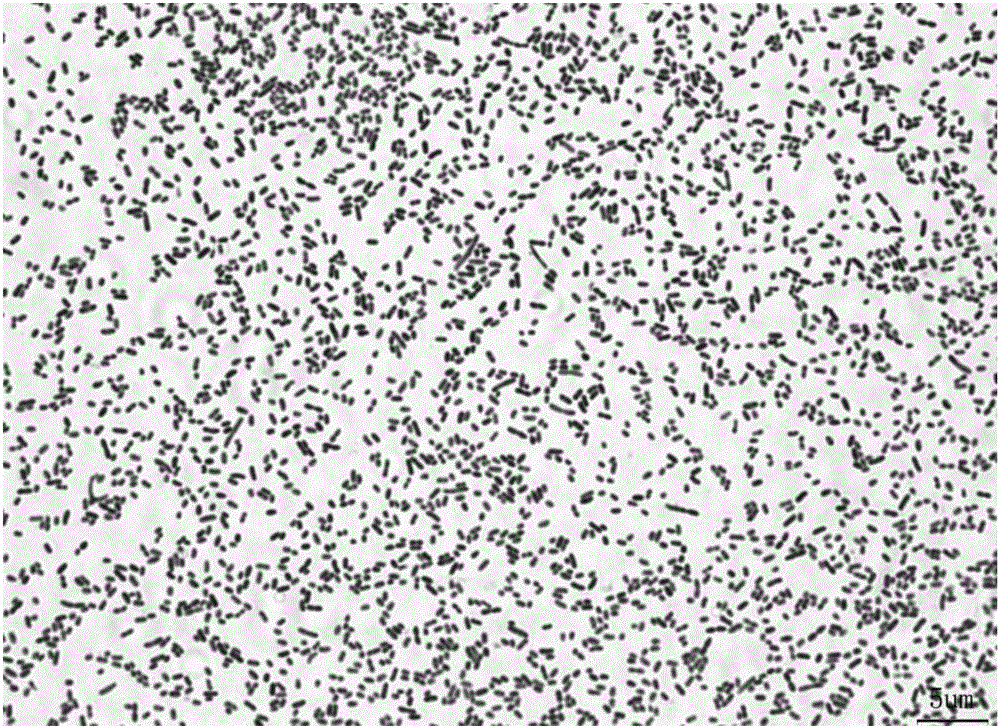
[0031] Isolation and identification of Aeromonas hydrophila HZ8:
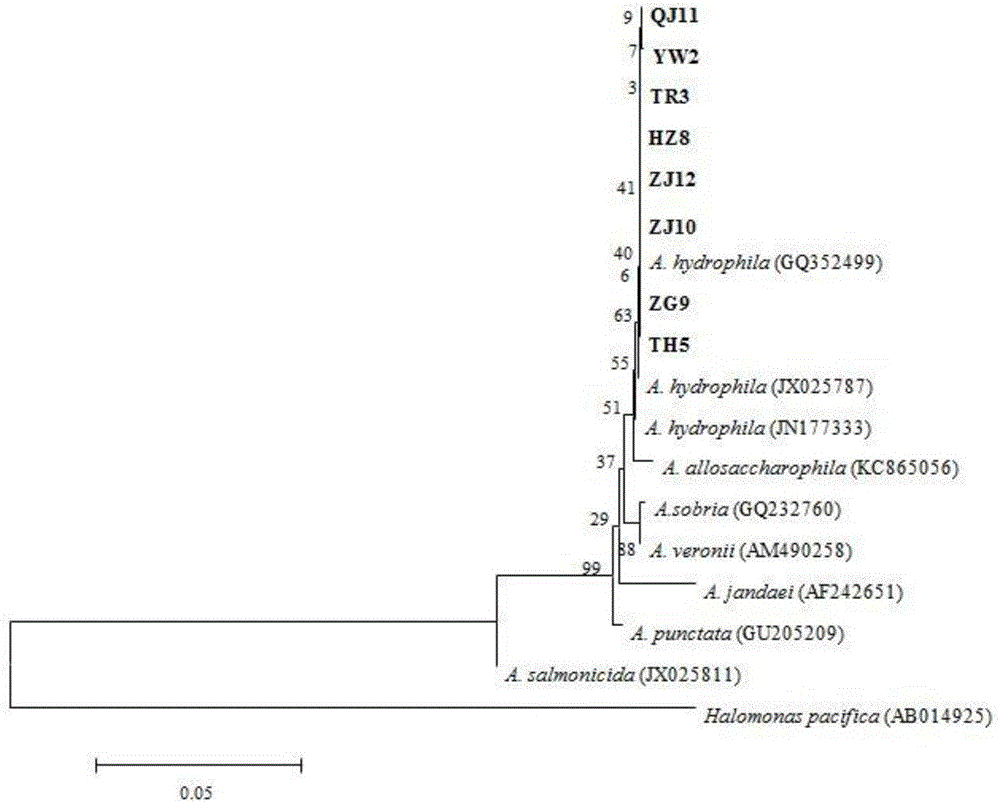
[0032] The present invention adopts the method of molecular biology to conduct experimental research on pathogenicity, phenotype analysis, molecular identification and virulence gene detection of eight strains of Sturgeon-derived Aeromonas hydrophila pathogenic bacteria. A phylogenetic tree was constructed based on the gyrB gene sequences of all strains, and the eight strains were all clustered with Aeromonas hydrophila; the distribution pattern of nine virulence genes in the strains was detected by PCR. Only the HZ8 strain contains all tested virulence genes: aerolysin (aer), flagellar gene (fla), heat-stable cell excitatory enterotoxin (ast), heat-unstable cell excitatory enterotoxin (alt), hemolysin (hly), elastase (ahyB), transverse flagellin (laf), enterotoxin (act) and lipase (lip). In addition, taking Sturgeon's sturgeon as the infection object, the half-lethal dose of HZ8 strain was the lowest, and the...
Embodiment 2
[0049] Preparation of immunogen and phagogen
[0050] Preparation of the immunogen:
[0051] Pick a single colony of Aeromonas hydrophila HZ8 in LB medium, culture at 37°C, 200 rpm for 12-16 hours.
[0052] Formalin is added to the cultured Aeromonas hydrophila bacteria solution to a final concentration of 0.3%, and inactivated in a constant temperature incubator at 37° C. for 48 hours, which is the inactivated vaccine of Aeromonas hydrophila. Centrifuge the inactivated vaccine at 5000r / min for 10min to precipitate the bacteria, suspend the bacteria in PBS and then centrifuge to precipitate the bacteria, repeat 3 times. Adjust the bacterial concentration to 1.0×10 8 CFU / ml, which is the immunogen, was stored in a refrigerator at 4°C for use in the following examples.
[0053] In the same way as above, the prepared inactivated Staphylococcus aureus was used as a phagogen, and it was stored in a refrigerator at 4°C for use in the following examples.
Embodiment 3
[0055] Safety detection of Aeromonas hydrophila HZ8 from sturgeon
[0056] (1) Plane sterility testing
[0057] Take 100 μL of the prepared immunogen, spread it on LB solid medium in a sterile operating bench, and incubate it in a constant temperature incubator at 37°C for 72 hours. Observation results show that there is no bacterial growth.
[0058] (2) Test fish safety testing
[0059] Take 30 healthy test fish (body length 15-20 cm), and immunize with the prepared immunogen by intraperitoneal injection, and the immunization dose is 1 ml / tail. Another 30 healthy test fish were intraperitoneally injected with the same dose of PBS as the control group. The test fish were fed normally and observed continuously for 14 days. There was no fish body death or typical symptoms after being infected by Aeromonas hydrophila.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com