A hylarana guentheri secretory peptide, a gene thereof and applications of the secretory peptide in pharmacy
A technology for secreting peptides and marsh water frogs, applied in the field of biomedicine, can solve the problem of less research on skin active peptide substances, and achieve the effect of convenient artificial synthesis, wide antibacterial spectrum and inhibiting bacteria.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1, the cloning of the secretory peptide gene of the marsh water frog:
[0030] 1. Extraction of total RNA from the skin of marsh water frog: the live marsh water frog was cleaned with water, put into liquid nitrogen and quick-frozen for 4h, got skin tissue, weighed, got 300mg skin tissue, added 10m total RNA extraction buffer (Trizol solution, the U.S. GIBCOBRL company product), homogenized in 20m1 glass homogenizer for 30min. Add an equal volume of phenol / chloroform solution, mix vigorously, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, and discard the precipitate. Add an equal volume of isopropanol to the supernatant, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, wash the precipitate once with 75% ethanol, and dry it.
[0031] II. Purification of marsh water frog skin mRNA: the separation and purification of marsh water frog skin mRNA was carried out by the American PROMEG...
Embodiment 2
[0043] Example 2, preparation of secreted peptide from marsh water frog:
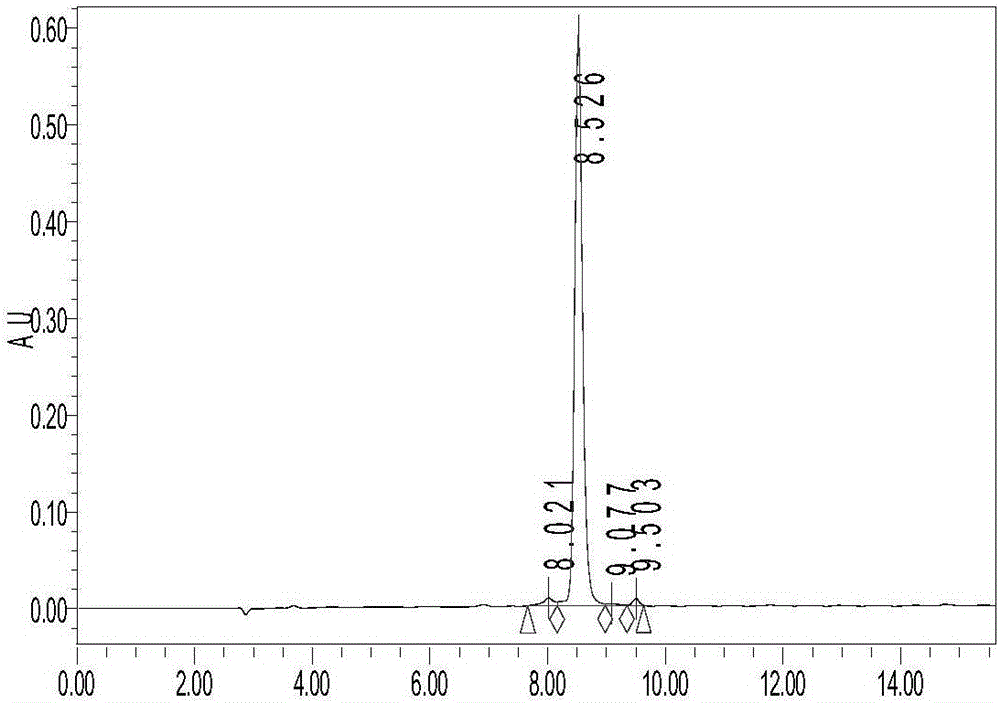
[0044] Ⅰ. The preparation method of the secreted peptide of the marsh water frog: according to the gene of the secreted peptide of the marsh water frog, the amino acid sequence of the mature living secreted peptide encoded by the function is deduced, and then the polypeptide is synthesized by an automatic polypeptide synthesizer. The formation of disulfide bond adopts the air oxidation method, specifically dissolving the polypeptide in the flask according to 0.1mg / ml in 0.1% acetic acid solution, titrating with ammonium hydroxide to pH 7.8, and then stirring overnight at room temperature. Desalted and purified by HPLC reverse phase C18 column chromatography. Liquid A is 0.05% TFA+2% CH during purification 3 CN, liquid B is 0.05% TFA+90% CH 3 CN, the polypeptide appears at 15 minutes, the concentration of solution B is 32-47%, and the detection wavelength is 220nm.
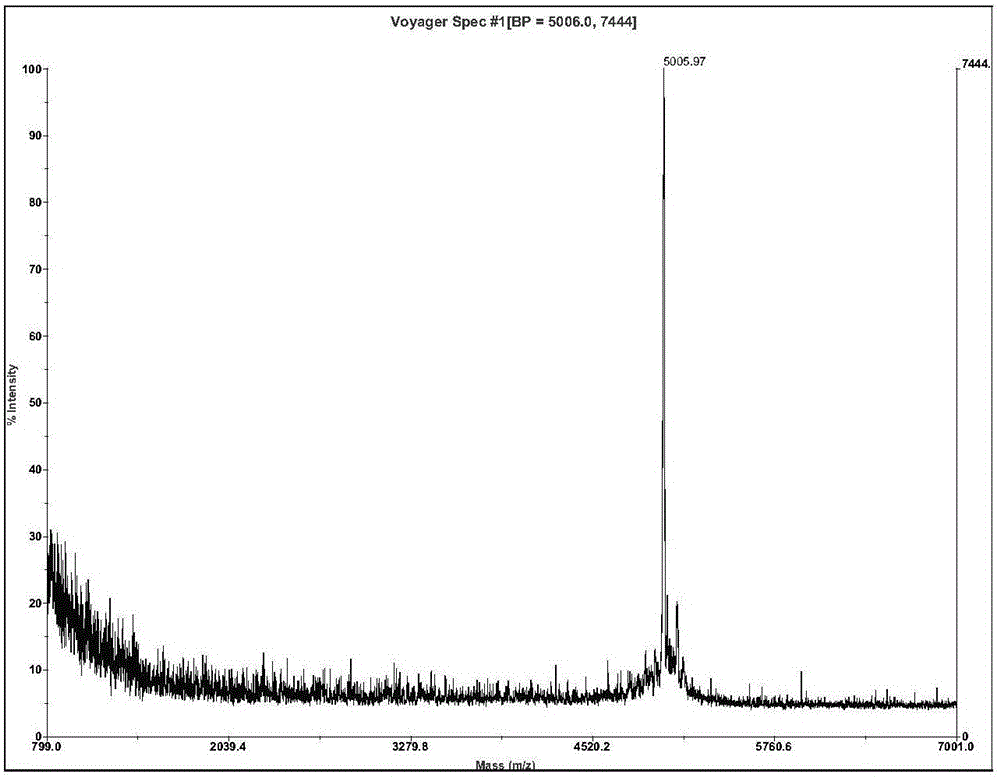
[0045] Ⅱ. The molecular weight is ...
Embodiment 3
[0048] Embodiment 3, the activity test of the secreted peptide of marsh water frog
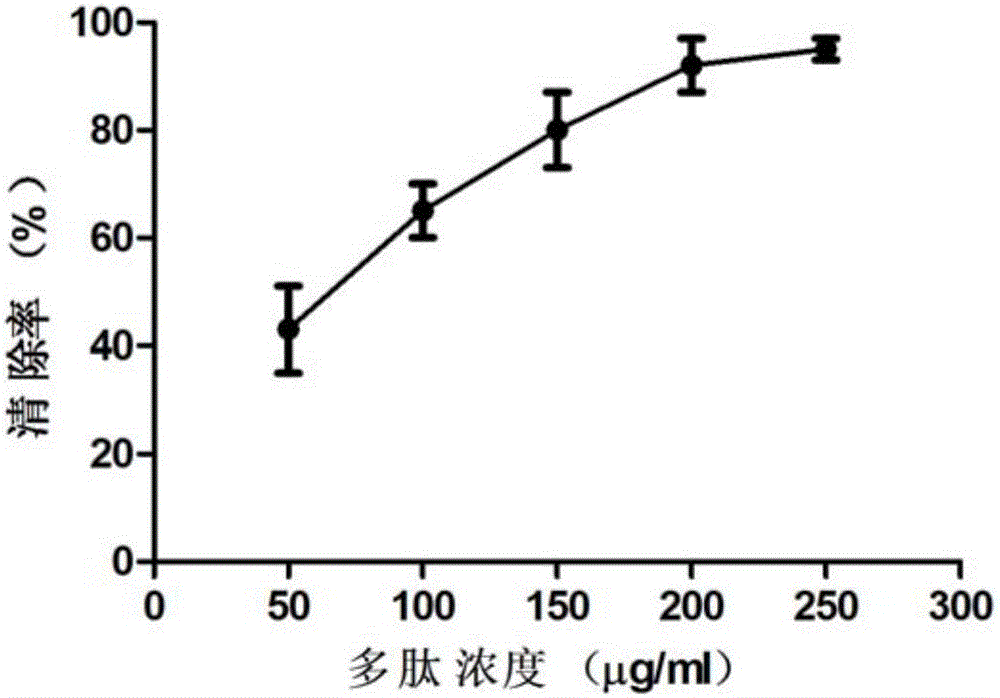
[0049] Ⅰ. Determination of the ability to inhibit bacterial growth
[0050] The antibacterial activity was detected by the cup and saucer method, and the medium was ordinary agar medium. Inject 20m1 of heated and melted culture medium into the plate as the bottom layer, spread it evenly in the bottom of the plate, after solidification, take another appropriate amount of culture medium and heat it to melt, then add 5m1 of bacterial suspension to each plate, shake well, Spread it evenly on the bottom layer as a bacterial layer. After cooling, put 6 sterilized stainless steel cups evenly in the plate at equal distances. Add 0.1 ml of the test compound solution at a concentration of 0.1-0.3 mg / ml to the first steel cup, add the sample solution to the remaining steel cups by doubling the dilution method, incubate at 37°C, and observe the size of the inhibition zone. The bacteriostatic zone above...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com