Treatment of severe hypertriglyceridemia
A technology for hypertriglyceridemia and hyperlipoproteinemia, which is applied in the field of treatment of severe hypertriglyceridemia, and can solve intractable and unreachable problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
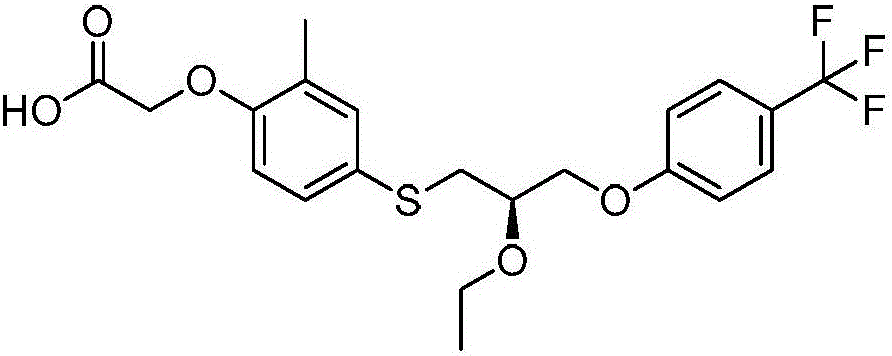
Image
Examples
Embodiment
[0061] This study is a 12-week interventional, open-label (single-blind), dose-escalation study using adult subjects (eg 30, preferably at least a quarter with type I hyperlipoproteinemia hyperlipoproteinemia and at least one quarter with type V hyperlipoproteinemia) with severe hypertriglyceridemia (fasting TG level of at least 1000 mg / dL) on stable therapy (fibrate, niacin, O3FA) or refractory to this therapy. Exclusion criteria included stage 3 or 4 heart failure, uncontrolled diabetes in the month before screening, use of corticosteroids in the month before screening, estrogen therapy (contraception or hormone replacement) (unless stable dose during the previous 2 months), pancreatic history during the 6-month period prior to screening, and current harvesting therapy. Subjects were assessed for fasting TG and other lipids at baseline. Subjects initially received 50 mg / day (when calculated as free acid) of MBX-8025 or MBX-8025 salt orally as a single daily dose for four w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com