Systemic delivery of virus vectors encoding urocortin-2 and related genes to treat diabetes-related cardiac dysfunction and congestive heart failure
A technology for urocortin and heart failure, applied in gene therapy, corticotropin releasing factor, skin diseases, etc., can solve problems such as adverse effects and weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Example 1: Intravenous delivery of AAV8 encoding urocortin-2 increases cardiac function in normal mice
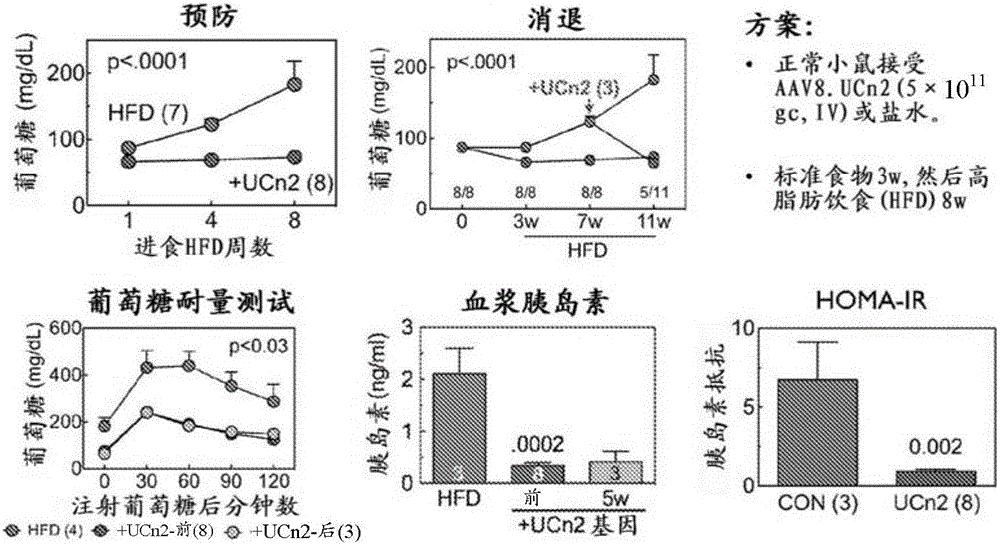
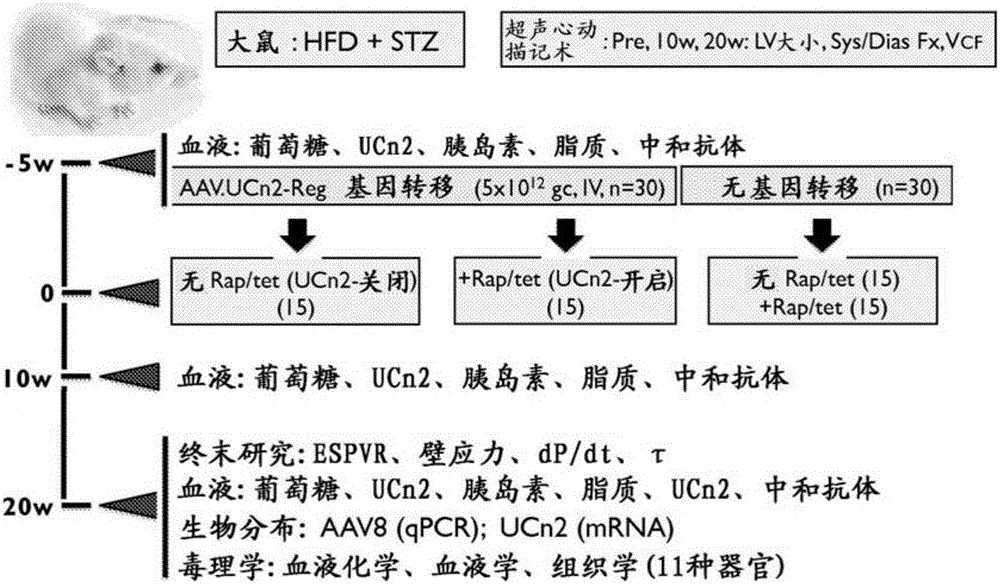
[0208] This example demonstrates the effectiveness of exemplary embodiments of the invention. In an alternative embodiment, adeno-associated virus vector serum encoding urocortin-2 (UCn2), a peptide of the corticotropin-releasing factor (CRF) family, using a single intravenous (IV) injection is provided. Type-8 (AAV8) composition and method for treating and improving type 2 diabetes mellitus (T2DM) and diabetic heart disease. In an alternative embodiment, the vector (AAV8.UCn2) comprises a regulated expression cassette to enable controlled expression. In alternative embodiments, exemplary vectors are delivered by intravenous injection, eg, into a brachial vein, during an outpatient visit.
[0209] We have demonstrated that a single intravenous injection of AAV8.UCn2 in mice resulted in a 15-fold increase in plasma UCn2 levels (which persisted for at least 7 month...
Embodiment 2
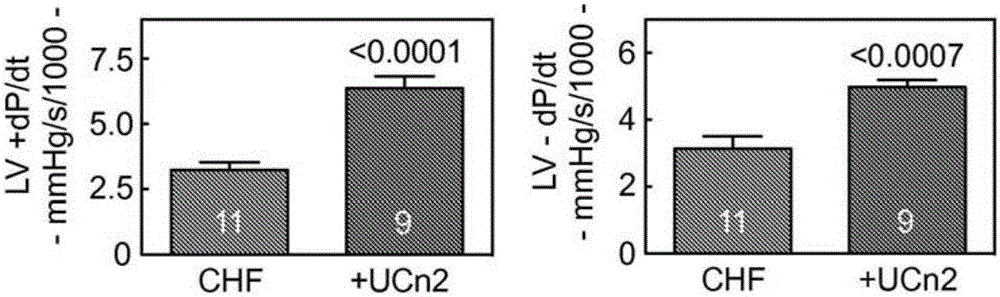
[0229] Example 2: Intravenous delivery of AAV8-encoded urocortin-2 increases function of failing hearts in mice
[0230] This example demonstrates the effectiveness of an exemplary embodiment of the invention that intravenous delivery of AAV8.UCn2 increases the function of failing hearts. In conclusion, myocardial infarction (MI, by coronary artery ligation) was used to induce heart failure, which was assessed by echocardiography 3 weeks after MI. Mice with LV ejection fraction (EF) 11 gc) or saline intravenous delivery, and echocardiography 5 weeks later showed increased LV EF in mice receiving UCn2 gene transfer (p=0.01). In vivo physiological studies showed a 2-fold increase in the peak rate of LV pressure rise (LV+dP / dt; p2+ The magnitude of the transient was associated with the rate of Ca+ decline and increased SERCA2a expression. Additionally, UCn2 gene transfer decreased Thr286 phosphorylation of Cam kinase II and increased expression of myomyosin light chain kinase,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com