Genetically engineered bacterium for producing L-aspartic acid through fermentation
A technology of genetically engineered bacteria and aspartic acid, applied in fermentation, enzymes, bacteria, etc., can solve problems such as deviations in the growth stability of strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This example illustrates the use of homologous recombination to knock out the genes encoding citrate dehydrogenase (icdA), genes encoding malate dehydrogenase (mdh), and genes encoding malic enzyme (sfcA and maeB) in the original Escherichia coli W1485 (ATCC12435) ) and the fumarase-encoding gene (fumAC) that function under aerobic conditions, the knockout process is basically the same. Taking the knockout of the fumAC gene as an example, the specific steps include:
[0046] 1. Using LB medium, cultivate Escherichia coli W1485 under aerobic conditions at 37°C to OD600=0.4-0.6, and make it competent for electroporation.
[0047] 2. Electrotransform the recombinant plasmid into competent Escherichia coli W1485. The electric shock conditions were: 200 Ω, 25 μF, electric shock voltage 2.3 kv, electric shock time 4-5 ms. Immediately after electric shock, the cells were added to pre-cooled 1 mL SOC medium, cultured at 150 r / min, 30°C for 1 h, and then spread on LB medium pla...
Embodiment 2
[0060] This example illustrates that mutant strains with good cell growth performance under aerobic conditions are obtained through evolutionary metabolic selection.
[0061] Evolutionary metabolism is the adaptation process of the bacteria itself to the environment. When a certain microorganism mutates during continuous culture, the mutant strain will compete with the original strain. If the mutant strain has an advantage over the original strain, the mutant strain will be in the reactor get reserved.
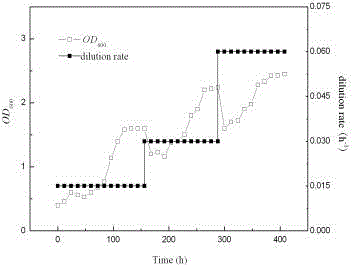
[0062] like image 3 As shown, the initial stage of continuous culture starts at 0.015 h -1 The dilution rate is continuously fed with fresh medium containing glucose. The density of the initial strain in the evolutionary metabolism device is 0.6. When the bulk density reaches a steady state and remains unchanged for a period of time, increase the dilution rate to 0.03 h -1 , the concentration of bacteria in the reactor drops rapidly, which indicates that the growth rate of...
Embodiment 3
[0064] This example illustrates the construction of an expression plasmid that overexpresses the gene encoding phosphoenolpyruvate carboxylase (ppc) and the gene encoding aspartase (aspA), and introduces the recombinant plasmid into the mutant strain CM-AS-105 , to increase the concentration and yield of strain L-aspartic acid.
[0065] 1. Construction of expression plasmids for overexpressing the gene encoding phosphoenolpyruvate carboxylase (ppc) and the gene encoding aspartase (aspA), the process including:
[0066] (1) Artificially design and synthesize an operon containing two genes with Nco I and Hind III restriction sites at both ends. See SEQ ID NO: 1 for the specific sequence.
[0067] (2) The expression plasmid pTrc99a was digested with Nco I and Hind III respectively, and ligated with the synthetic gene to obtain the recombinant plasmid pTrc99a-ppc-aspA.
[0068] 2. The plasmid pTrc99a-ppc-aspA was introduced into the competent mutant strain CM-AS-105, and the posi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com