Club moss alkaloid compound and medicine composition therefore, as well as preparation method and application thereof
A technology of lycopodium alkaloids and compounds, which is applied in the directions of drug combination, organic chemistry method, pharmaceutical formula, etc., can solve the problem of no preparation method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
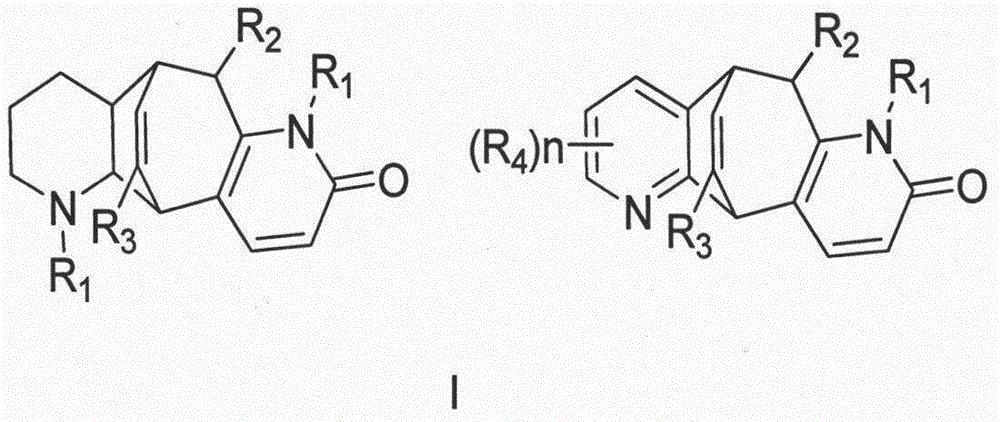
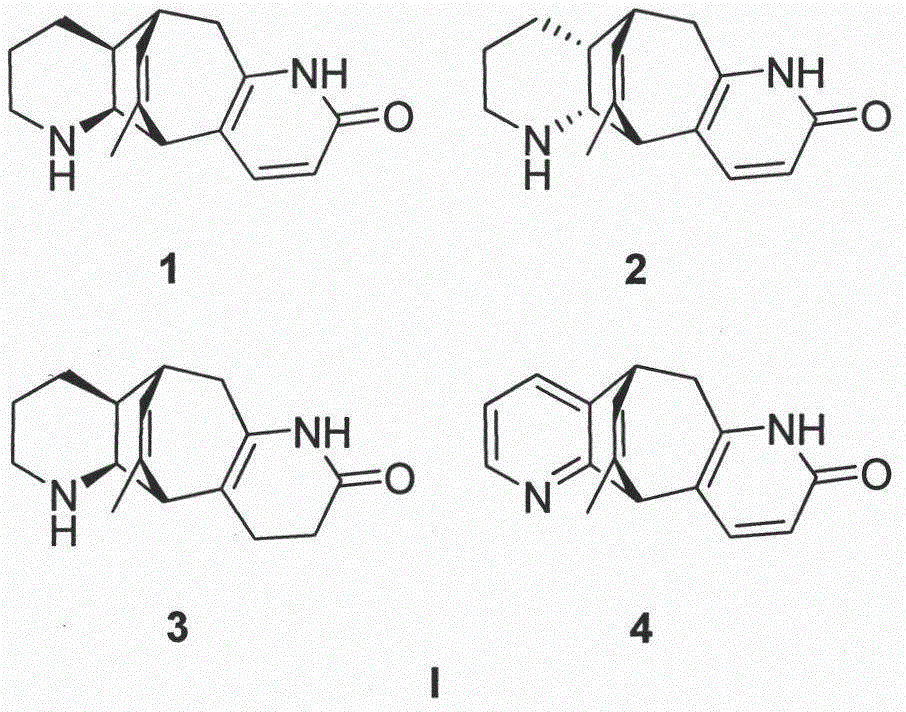
[0040] Preparation method and structure identification of lycopodium alkaloids phleghenrines A-D(1-4):
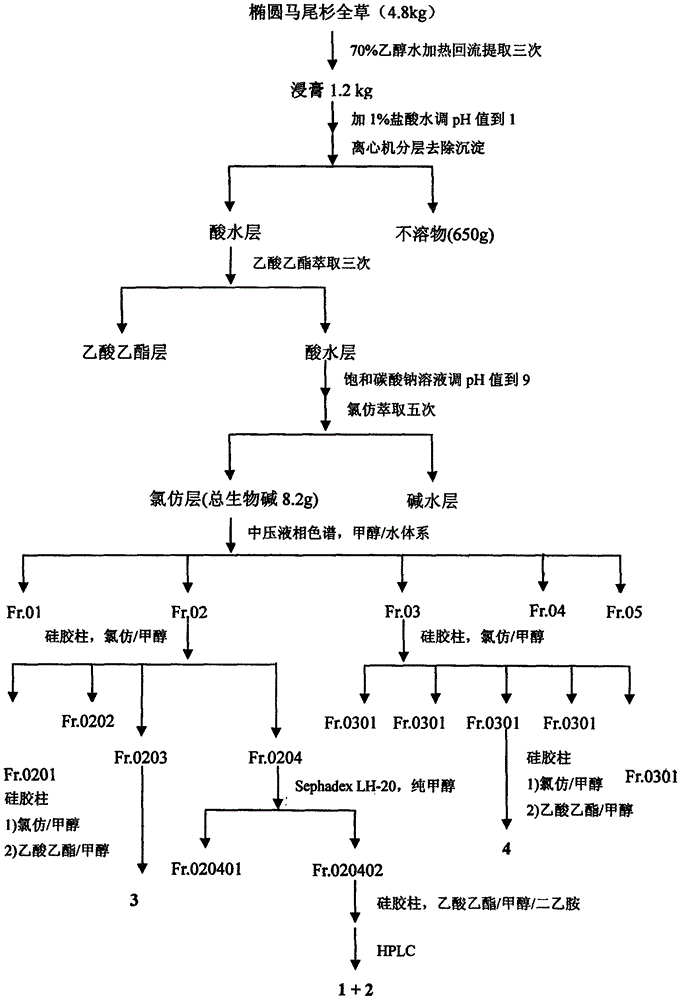
[0041] Get 4.8Kg of dry whole herb of Phlegmariurus henryi (Baker) Ching, grind it and extract it under reflux with 70% industrial ethanol / water mixed solvent (60L), extract three times altogether, the first time is 4 hours, and the last two times each time After 3 hours, the combined extracts were evaporated and concentrated under reduced pressure to remove the organic solvent to obtain a total of 1.2Kg of extract. The lower layer insoluble matter (650g), and then the upper layer aqueous solution was extracted three times with ethyl acetate, 8L each time, to remove most of the non-alkaloid components. The extracted water-soluble part was adjusted to pH 9 with saturated sodium carbonate solution, and then fully extracted with chloroform five times, 8 L each time, to obtain 8.2 g of the total alkaloid extract. The extract was mixed with 50-mesh polyamide (20g), dried and pa...
Embodiment 2
[0054] The phleghenrines A-D (1-4) of the lycopodium alkaloid compound of the present invention shows obvious inhibitory activity in inhibiting acetylcholinesterase. The experimental principles, methods and results are as follows:
[0055] Experimental principle: Acetylcholinesterase can catalyze the degradation of its substrate analogue, thioacetylcholine iodide, to generate thiocholine and acetic acid, and the reaction product reacts with the chromogenic agent DTNB to generate a yellow substance, which has specific light absorption at 405nm. The mixture of compound and acetylcholinesterase reacts at 30°C. If the compound has an inhibitory effect on acetylcholinesterase, the amount of acetylcholinesterase catalyzing the degradation of the substrate analogue thioacetylcholine iodide will decrease, and the corresponding reaction with DTNB will generate The reduction of the yellow compound, that is, the light absorption value at 405nm becomes smaller, so as to screen the compoun...
Embodiment 3
[0067] Compound 1-4 obtained in Example 1 was added with 4% sulfuric acid ethanol solution, pH = 4, filtered and dried to prepare sulfate compound 1-4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com