A kind of thiazole bisamide compound and its preparation method and application
A technology of thiazole bisamide and compound is applied in the field of novel thiazole bisamide compound and its preparation, and achieves the effect of good bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
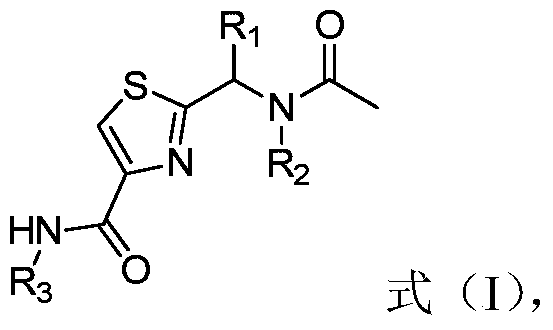
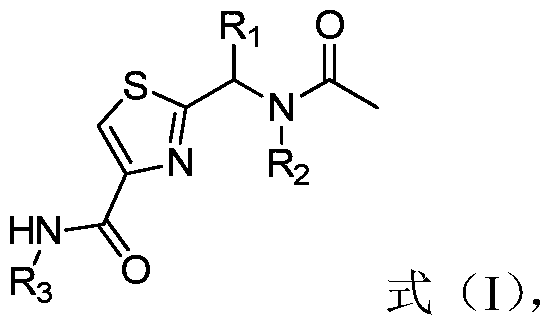
[0033] In a second aspect, the present invention provides a method for preparing thiazole bisamides, the method comprising the following steps:
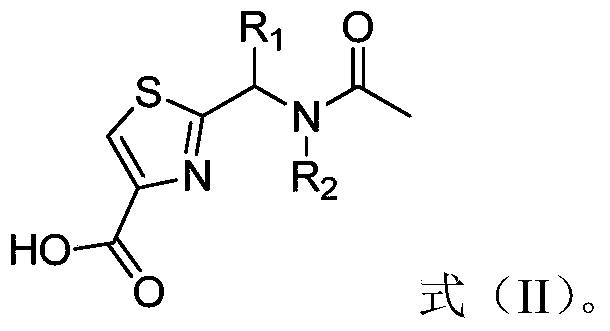
[0034] (1) The intermediate shown in formula (II), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1-hydroxybenzotriazole and N,N- Dimethylformamide for the first contact;
[0035] (2) put R 3 -NH 2 Shown intermediate carries out second contact with the product after step (1), wherein, R 3 is a substituted or unsubstituted diphenyl ether group, 2-biphenyl, 3-biphenyl, 4-biphenyl or phenyl; R 3 Substituents in include fluorine, chlorine, bromine, iodine, methyl, ethyl, n-propyl, isopropyl, cyclopropyl, methoxy, ethoxy, n-propoxy, isopropoxy and cyano At least one of the radicals and methyl, ethyl, n-propyl, isopropyl, cyclopropyl, methoxy, ethoxy, n-propoxy and isopropoxy substituted by halogen elements at least one; and
[0036] (3) purifying the product after step (2);
[0037] where, in formula (II), R 1 is ...
preparation example 1-5
[0082] This preparation example is used to illustrate the preparation of the intermediate represented by formula (III) according to the method I of the present invention.
[0083] Under nitrogen protection, the R 1 The reactant (10mmol) shown in -CHO, the reactant (10mmol) shown in the formula (IV), the reactant (10mmol) shown in the formula (V) and the reactant (10mmol) shown in the formula (VI) dissolve In 10mL of anhydrous methanol, react at a temperature of 25°C for 24 hours; after the reaction is complete, remove the solvent from the reaction product under reduced pressure and directly use it in the next reaction;
[0084] The crude product obtained in the previous step was dissolved in 10 mL of trifluoroacetic acid, and reacted at 50°C for 12 hours; after the reaction was complete, the solvent was removed under reduced pressure, and purified by column chromatography to obtain product intermediates marked as III-1~III-5 .
[0085] Among them, R 1 As shown in Table 1;
...
preparation example 6-20
[0092] This preparation example is used to illustrate the preparation of the intermediate represented by formula (III) according to the method II of the present invention.
[0093] Under nitrogen protection, the R 1 The reactant (10mmol) represented by -CHO, R 2 -NH 2 The reactant (10mmol) shown, the reactant (10mmol) shown in formula (V) and the reactant (10mmol) shown in formula (VI) were dissolved in 10mL of anhydrous methanol and reacted at a temperature of 25°C for 24h ; After the reaction is complete, the reaction product is desolvated under reduced pressure, and purified by column chromatography to obtain product intermediates marked as III-6~III-20.
[0094] Among them, R 1 and R 2 As shown in table 2;
[0095] Specifically, the reaction process is shown in reaction formula (4); and
[0096] Results The prepared products and their yields are shown in Table 2.
[0097] Table 2
[0098] serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com