Preparing method and application of oral insulin transport system in layer-by-layer self-assembly structure
A layer-by-layer self-assembly and insulin technology, which is applied in the field of preparation of oral insulin delivery system, can solve the problems of insulin deficiency and poor insulin effect, and achieve the effects of low toxicity, control of blood sugar level, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Preparation and Characterization of Oral Insulin Delivery System (LBL) with Layer-by-Layer Self-Assembled Structure
[0088] 1. Preparation of LBL particles
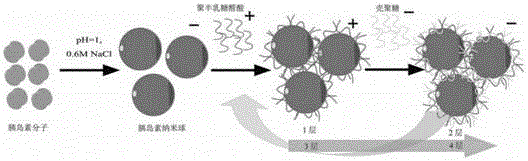
[0089] The synthetic method of LBL particle is as attached figure 1 shown.
[0090] (1) Dissolve 5mg of insulin in 1ml of dilute hydrochloric acid solution with pH 1.1 at 15°C, then add solid NaCl to make the final concentration of NaCl reach 0.6M, stir the solution for 0.5h, and centrifuge at 5000rpm for 3min to obtain a precipitate;
[0091] (2) Add the polygalacturonic acid (PGLA) solution of pH = 4, NaCl 0.6M to the precipitate in step (1), stir for 0.5 h, sonicate for 1 min, continue stirring for 0.5 h, and centrifuge at 5000 rpm for 3 min to obtain the precipitate;
[0092] The preparation method of the polygalacturonic acid (PGLA) solution of described pH=4, NaCl 0.6M is: polygalacturonic acid (PGLA) is dissolved in 1ml dilute hydrochloric acid solution, then adds NaCl solid, makes NaCl final co...
Embodiment 2
[0121] Example 2 Cell experiment
[0122] 1. Experimental method
[0123] (1) Cell culture
[0124] Caco-2 cell line was provided by Guangdong Pharmaceutical University. After the cells were proliferated and cultured to 80% in the culture flask, they were seeded on a 96-well plate at a density of 5000 / well, and cultured for 1 or 2 days for subsequent experiments. The cell culture conditions are: high-glucose DMEM medium containing 20% newborn calf serum, non-essential amino acids, 37°C, 5.0% CO 2 .
[0125] (2) Transport system toxicity
[0126] Different doses of the transport system were added to the Caco-2 cells being cultured, and after 24 hours, the cell viability was measured by the MTT method. That is, inoculate Caco-2 cells on a 96-well culture plate, 5 000 cells / well, after 24 hours of culture, aspirate the medium, add serum-free medium, and different concentrations of insulin delivery system, after 24 hours of culture, add 5mg / ml MTT solution 20ul / well. Cont...
Embodiment 3
[0130] Example 3 In Vivo Experiment—Studies on Cytotoxicity of the System
[0131] 1. Construction of type I diabetic mouse model
[0132] (1) Method
[0133] Purchase 6-week-old SD rats and adapt to the environment for one week. Take 3 as the control group, and the rest as the experimental group. Rats were fasted overnight, blood glucose was measured, and STZ solution was prepared with a concentration of 15 mg / ml by using citrate buffer solution with pH=4.4 in ice bath and protected from light before injection. Rats in the experimental group were injected intraperitoneally at a dose of 50 mg / kg.
[0134] From the day of modeling, the body weight, food intake and water intake of the rats were measured daily. On the 3rd and 7th day, the blood glucose was monitored with the Roche active blood glucose meter. For rats with low blood sugar, supplementary injection of STZ (30mg / kg).
[0135] One week later, those whose fasting (after 3 h of starvation) blood glucose concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com