Astragaloside peritoneal dialysis solution
An astragaloside IV and glycoside peritoneal technology, which is applied in the field of medicine, can solve the problems of many adverse reactions, many impurities, complex components, etc., and achieve the effects of wide clinical application value, improving cell metabolism and reducing renal fibrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0041] Embodiment two: Observing the effects of astragaloside IV peritoneal dialysis solution and conventional peritoneal dialysis solution on the efficacy of peritoneal dialysis in rats through animal experiments
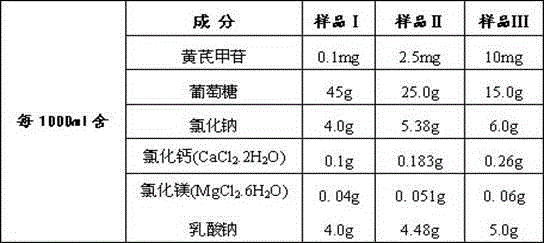
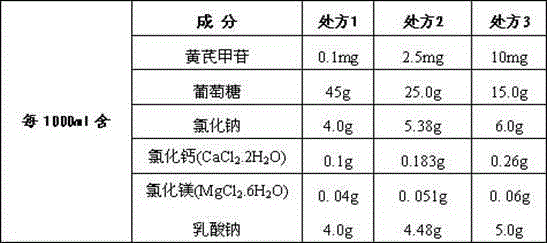
[0042] sample: The astragaloside IV peritoneal dialysate sample prepared by the method of embodiment one Ⅰ ,sample Ⅱ ,sample Ⅲ :
[0043] Control: Peritoneal Dialysis Solution (Lactate-G1.5%) Shanghai Changzheng Fumin Jinshan Pharmaceutical Co., Ltd.
[0044] ReagentUrea Oxygen (BUN), Creatinine (Cr) and Protein Determination Kit Beckman Company, USA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com