A bacillus amyloliquefaciens nitrite reductase, a gene and applications
A technology for decomposing starch spores and nitrite, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Embodiment 1: Cloning and expression of Bacillus amyloliquefaciens H47 nitrite reductase gene, the steps are as follows:
[0038] Bacillus amyloliquefaciens H47 bacterial genome total DNA was extracted according to its operation using a bacterial genomic DNA extraction kit (TaKaRa Company), its quality and purity were identified by agarose gel electrophoresis, and its concentration was determined by an ultraviolet spectrophotometer. Design upstream and downstream primers based on whole gene sequencing: m1 (SEQ ID NO: 3,5'-GCCGCATATGATGGGAAAAAAACAGCTAGTC-3') and m2 (SEQ ID NO: 4,5'-GCCGGGATCCTCAATACAGGATGTATACATTCTC-3'), using the PCR method, using the above-mentioned Bacillusamyloliquefaciens The H47 genome was used as a template, and the above-mentioned m1 and m2 were used as specific primers to amplify the full-length coding frame sequence of the Bacillusamyloliquefaciens H47 nitrite reductase gene. The PCR reaction conditions are: Genomic DNA is used as a template, in...
Embodiment 3
[0049] Example 3: Extraction, purification and enzyme activity determination of crude protein in recombinant bacteria
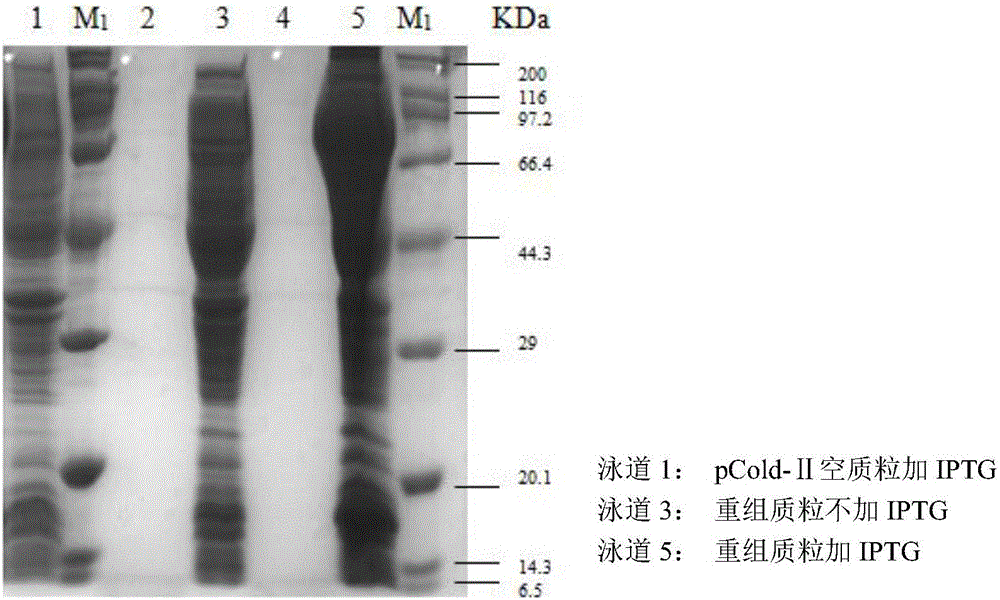
[0050] The recombinant thallus obtained in Example 2 and the control thallus are washed twice with sterile water, add 10 ml per 100 ml of culture solution, and pre-cool 0.02M phosphate buffer (pH7.0) in an ice bath at 4°C Ultrasonic crushing for 5 minutes, after crushing, centrifuge at 8000rpm at 4°C for 5 minutes, take the supernatant as NiR crude protein solution, and perform SDS-PAGE detection at the same concentration, the results are as follows: image 3 As shown, lane 1 is the crude enzyme solution of BL21 group containing empty plasmid induced by adding IPTG, lane 3 is the crude enzyme solution of BL21 group containing recombinant plasmid induced by adding IPTG, and lane 5 is the crude enzyme solution of BL21 group containing recombinant plasmid induced by adding IPTG The crude enzyme solution shows that there are two obvious specific expression bands ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com