Method for detecting sorafenib-tosylate-related substances
A technology related to substances and detection methods, applied in the field of analytical chemistry, can solve problems such as poor resolution, impurity inspection, system applicability, repeatability, linear range, quantitative limit and detection limit difference, etc., to achieve high system adaptability, The effect of high precision and high peak symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: chromatographic conditions and chromatographic system of detection method of the present invention
[0041] Instrument: Shimadzu high performance liquid chromatography
[0042] Chromatographic column: Waters Symmetry C18 (4.6×100mm, 3.5μm)
[0043] Mobile phase A: pH3.0 phosphate buffer (take 2.72g potassium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, adjust the pH value to 3.0 with phosphoric acid); mobile phase B: acetonitrile:ethanol=60:40; detection wavelength : 250nm; Flow rate: 1.0ml / min; Injection volume: 20μl; Column temperature: 35℃
[0044] Table 1: Elution Program
[0045] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
65
35
10
50
50
26
50
50
27
65
35
45
65
35
[0046] Preparation of the test solution: Get about 27.4 mg of Sorafenib tosylate, put it in a 100ml measuring bottle, add an appropriate amount of mixed solution, ultrasonical...
Embodiment 2
[0049] Embodiment 2: detection method system applicability detection of the present invention
[0050] Diluent: mobile phase A-mobile phase B (1:3) (V / V); blank solution: diluent
[0051] Impurity stock solution: Take about 20 mg each of impurity A, impurity B, impurity C, impurity D, impurity E and impurity F, weigh them accurately, put them in a 100ml measuring bottle, add an appropriate amount of diluent, dissolve them by ultrasonic, let cool, and dilute with Dilute to the mark with diluent, shake well, accurately measure 5ml, put it in a 50ml measuring bottle, dilute to the mark with diluent, shake well, and use it as the reference substance stock solution;
[0052] System suitability test solution: take another about 27.5 mg of sorafenib tosylate reference substance, accurately weigh it, put it in a 100ml measuring bottle, add an appropriate amount of diluent, dissolve it by ultrasonication, then precisely add 1ml of the above stock solution, and dilute it with Dilute th...
Embodiment 3
[0057] Embodiment 3: Specific detection of the detection method of the present invention
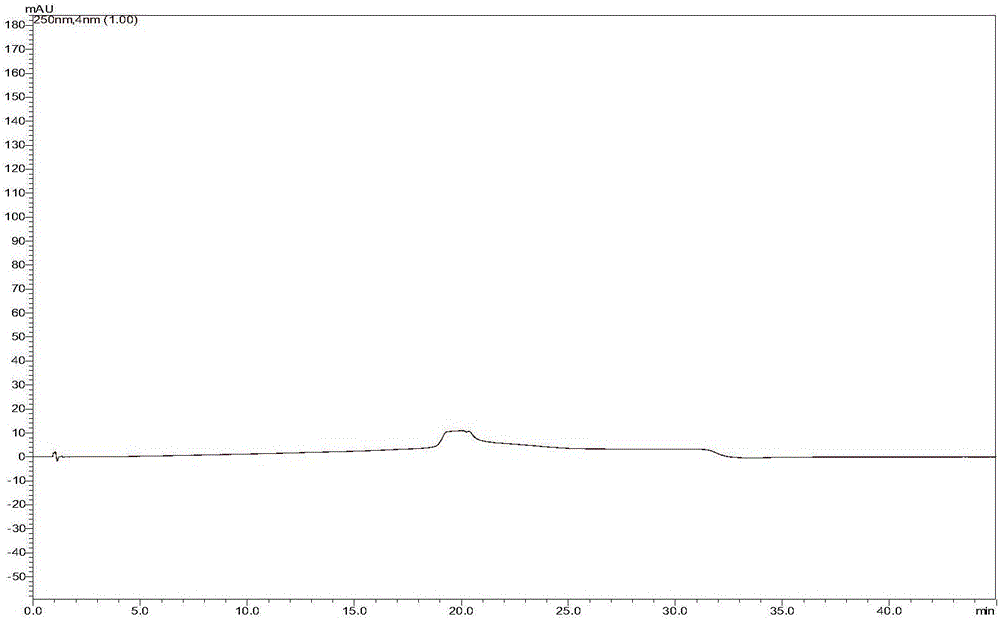
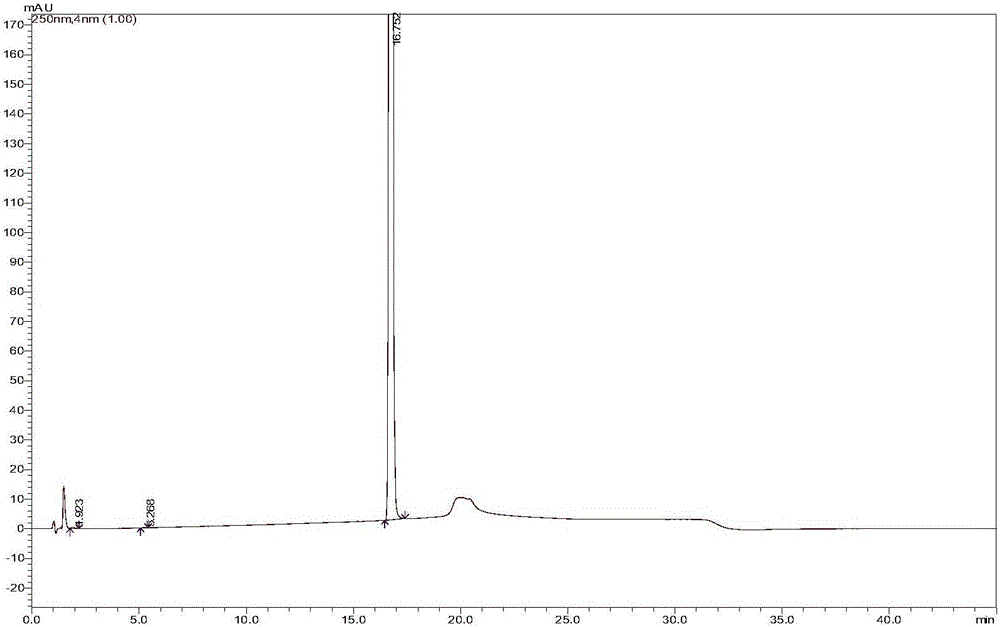
[0058] The strong degradation test is to simulate the strong degradation conditions of strong acid, strong alkali, oxidation, high temperature and light to destroy sorafenib tosylate. The effectiveness and applicability of the analytical method can be evaluated by comparing the amount of impurity generated with the reduction of the main component. At the same time, the DAD detector is used to check the peak purity: in the spectrum obtained from the degradation test, when the purity factor of the main component is greater than the threshold, it can be judged that the chromatographic peak does not contain other impurity peaks, and the chromatographic peak purity meets the requirements. The specific test results are shown in the table 4. The corresponding chromatogram instructions are attached Figure 3-8 .
[0059] Table 4: Specificity test results
[0060]
[0061] According to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com