Preparation method of tumor immune antigen, and products and application of preparation method
一种免疫抗原、肿瘤的技术,应用在医学工程领域,能够解决肿瘤治疗效果不佳等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
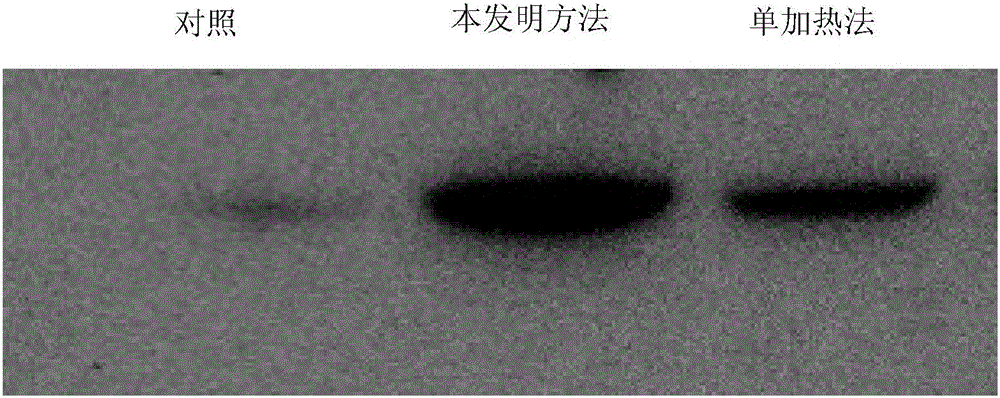
[0096] The present invention also provides a method for tumor immune antigen (especially HSP70), the method is based on the sequential cooling-heating treatment of the present invention, after cold-heating the tumor cells cultured in vitro, separate and purify the tumor cell culture medium tumor immune antigens. Preferably, the preparation method of the present invention comprises:
[0097] A) cooling the cultured tumor tissue and / or tumor cells to obtain cooled tumor tissue and / or tumor cells; wherein, the cooling includes cooling the tumor tissue and / or tumor cells to T1, and -50°C≤T1≤0°C, preferably, -30°C≤T1≤-10°C, more preferably, -25°C≤T1≤-15°C; most preferably, -20°C≤T1≤-18°C ;
[0098] B) heating the cooled tumor tissue and / or tumor cells obtained in A), so as to obtain tumor tissue and / or tumor cell cultures containing tumor immune antigen groups; wherein, the heating includes obtaining in A) The cooled tumor tissue and / or tumor cells are heated to T2, and 37°C<T2≤...
Embodiment 1
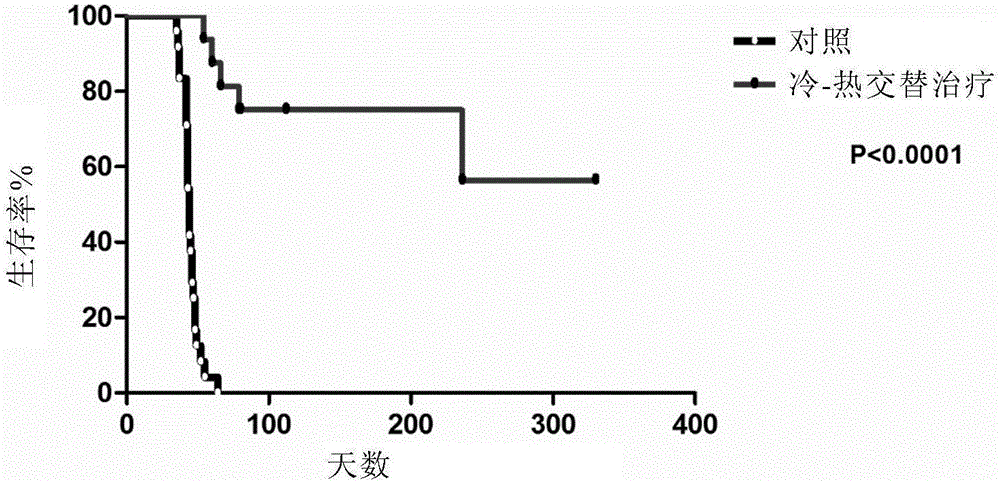
[0133] Embodiment 1 cold-heat treatment improves the survival rate of mice
[0134] The mice in the cold-heat treatment group (n=16) were treated with cold-heat treatment, wherein the temperature was lowered to -20°C within 10 minutes, the temperature was lowered for 5 minutes, and the temperature was heated to 50°C within 10 minutes for 20 minutes.
[0135] The mice in the blank control group (n=16) did not receive treatment.
[0136] Observe the living conditions of the cold-heat treatment group and the blank control mice for one year ( figure 1 ). Sixteen of the cold-heat treated mice survived longer than 66 days after inoculation, and finally 11 mice survived to one year. However, the survival time of the 16 mice in the control group did not exceed 64 days after inoculation.
Embodiment 2
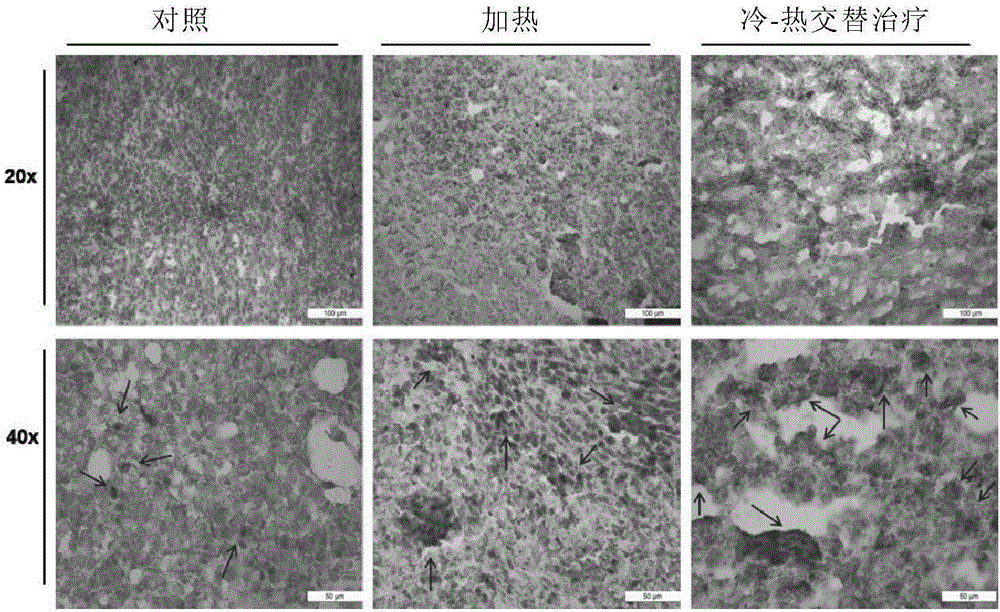
[0137] Example 2 Cold-Heat Therapy Promotes the Release of HSP70 from Local Tumors
[0138] After 1 day of cold-heat treatment and single heat treatment in Example 3, and 22 days after inoculation, orthotopic tumor sections were taken for immunohistochemistry. ( figure 2 ) Brown (light color) is the specific staining of HSP70 protein by the antibody, and blue (dark color) is the staining of the cell nucleus by hematoxylin.
[0139] In the control group, it was seen that some isolated HSP70 existed, most of which existed in the cells and were not released. This kind of HSP70 mainly played a role in promoting the survival of tumor cells.
[0140] Regional release of HSP70 could be observed in the single heat treatment group and combined cold and heat treatment group, but in the single heat group, the release area of HSP70 was relatively limited, and some areas with intact nuclei did not release HSP70;
[0141] In the cold-heat treatment group, necrotic death and cell fragme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com