Benzothiazole 2-acetonitrile dye and application thereof
A technology of benzothiazole and acetonitrile, applied in styryl dyes, organic dyes, methine/polymethine dyes, etc., can solve the problems of easy quenching and lack of aggregation-induced luminescence effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of benzothiazole 2-acetonitrile dyes
[0026] Add 2.73g (10mmol) 4-(diphenylamine) benzaldehyde, 1.74g (10mmol) benzothiazole 2-acetonitrile-2-acetonitrile and 0.77g (10mmol) ammonium acetate in a 50mL flask, and then add 20mL absolute ethanol. After reacting overnight at room temperature, the precipitate was filtered and recrystallized in ethanol to obtain 3.56 g of an orange-red solid. Yield 83%.
[0027] 2. Compound Characterization
[0028] 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.01(s,1H),7.94(d,1H),7.78(t,3H),7.39(t,1H),7.25(m,5H),7.08(m,6H),6.93( d, 2H).
[0029] 13 C NMR (100MHz, CDCl 3 )δ(ppm):163.91,153.75,151.58,146.18,145.98,134.79,132.26,129.78,126.75,126.30,125.49,125.23,124.47,123.16,121.59,119.72,117.55,100.71,77.43,77.12,76.80.
[0030] IR (cm -1 ,KBr):3750,3056,2360,2333,2205,1700,1566,1506,1482,1426,1331,1295,1164,1069,980,914,819,754,724,700,617,587,533,498
[0031] HR-MS (ESI): C 28 h 19 N 3 S m / z,429for[M+Na] + :452.1203
[0...
Embodiment 2
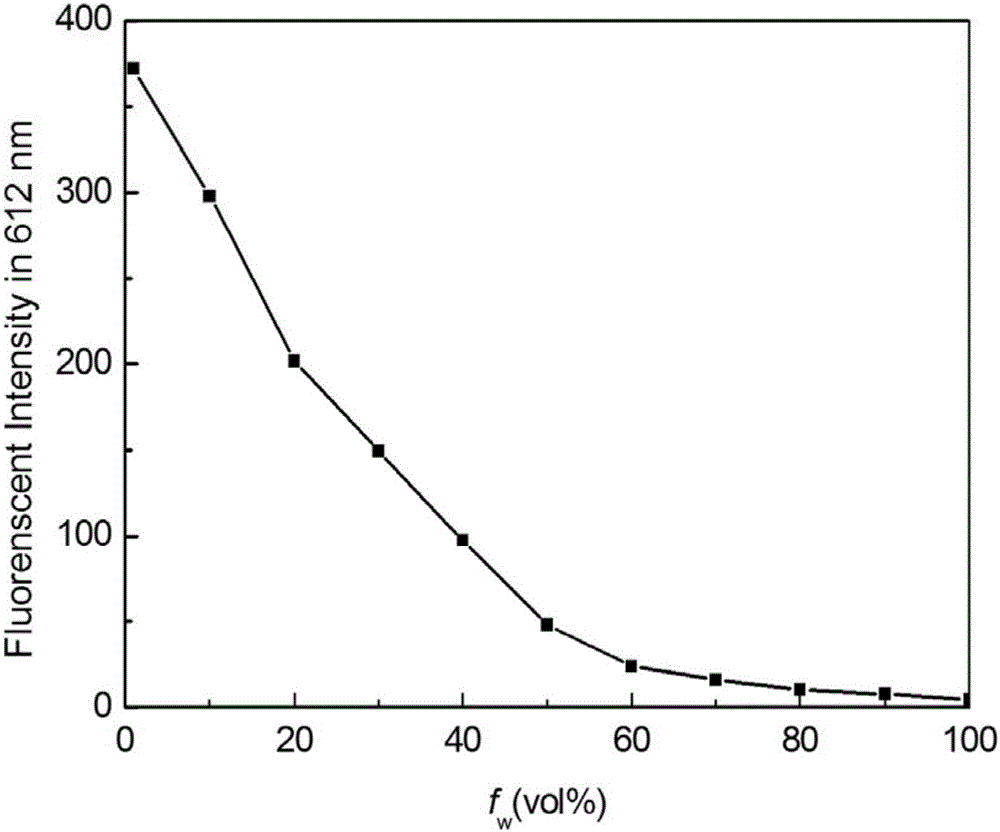
[0035] Embodiment 2 (fluorescent properties of benzothiazole 2-acetonitrile dyes)
[0036]Prepare 5 mM benzothiazole 2-acetonitrile dye DMSO solution, take 10 μL benzothiazole 2-acetonitrile dye DMSO solution, add 10 mL volumetric flask, add 1, 2, 3, 4, 5, 6, 7 , 8, 9mL of distilled water, and then add DMSO to adjust the volume of the solution to 10mL to obtain a benzothiazole 2-acetonitrile dye water / DMSO solution (9 / 1, v / v) with a concentration of 1 μM and a benzothiazole with a concentration of 5 μM 2-acetonitrile dye water / DMSO solution (8 / 2, v / v), the concentration of benzothiazole 5 μM 2-acetonitrile dye water / DMSO solution (7 / 3, v / v), the concentration of 5 μM benzene Benzothiazole 2-acetonitrile dye water / DMSO solution (6 / 4, v / v), the concentration is 5 μM Benzothiazole 2-acetonitrile dye water / DMSO solution (5 / 5, v / v), the concentration is 5 μM The benzothiazole 2-acetonitrile dye water / DMSO solution (4 / 6, v / v), the concentration of 5 μM benzothiazole 2-acetonitrile ...
Embodiment 3
[0044] The following benzothiazole 2-acetonitrile dyes represented by the chemical formula (I) are used as fluorescent probes for detecting cyanide ions.
[0045] 1. Fluorescent detection of cyanide ion
[0046] 1. Selectivity of Fluorescent Detection of Cyanide Ion
[0047] (1) Prepare an acetonitrile solution of benzothiazole 2-acetonitrile dye with a concentration of 5 mM.
[0048] (2) Prepare NaF, NaCl, KBr, KI, tetra-n-butylammonium cyanide, CH 3 COONa,NaNO 3 , Na 2 SO 4 ,NaHSO 4 ,NaHSO 3 ,NaH 2 PO 4 of aqueous solution.
[0049] (3) Take 10 μL of benzothiazole 2-acetonitrile dye acetonitrile solution, add dropwise NaF, NaCl, KBr, KI, tetra-n-butylammonium cyanide, CH 3 COONa,NaNO 3 , Na 2 SO 4 ,NaHSO 4 ,NaHSO 3 ,NaH 2 PO 4 10 μL of the aqueous solution of benzothiazole 2-acetonitrile dye was diluted to 10 mL with water, and the fluorescence change of the benzothiazole 2-acetonitrile dye aqueous solution was observed under the excitation of 365 nm light. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com