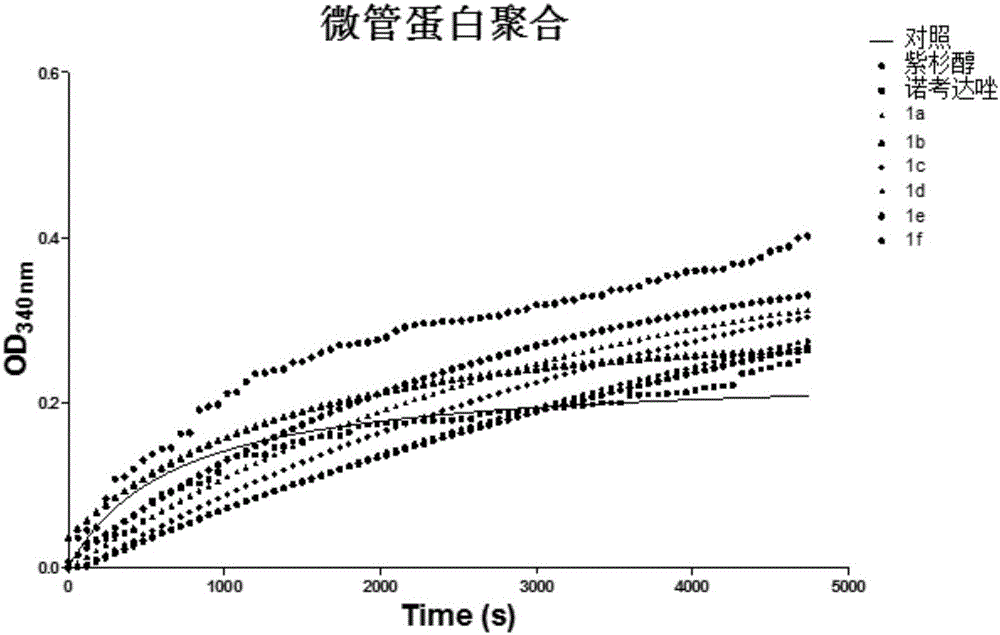
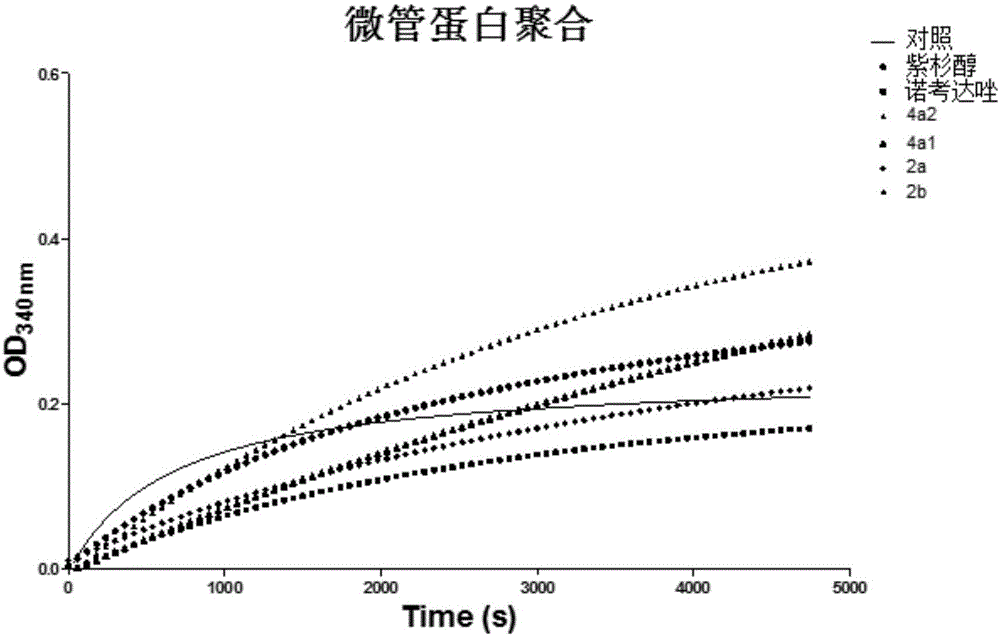
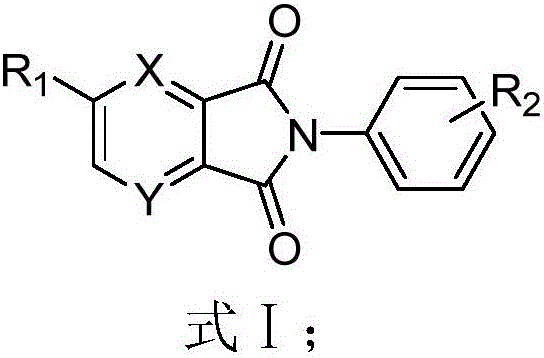
Diketone derivative as well as preparation method and application thereof
A technology of derivatives and diketones, which is applied in the field of diketone derivatives and its preparation, can solve problems affecting the polymerization process of tau protein, protect nerve cells, prevent tubulin depolymerization, and treat Alzheimer's disease Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 5-bromo-2-(2,6-diisopropyl-phenyl)-isoindole-1,3-dione 1a
[0033] Weigh 4-bromophthalic anhydride (0.67mmol, 1eq) and compound 2,6-diisopropylaniline (0.80mmol, 1.2eq) in a 25mL round bottom bottle, dissolve it in acetonitrile (3mL) After that, add DPPOx (1.34mmol, 2eq) prepared by reference (Tetrahedron, 1982,39 (20): 3253), Et 3 N (0.18mL, 1.29mmol, 1.9eq), the reaction continued for 2.5h under the condition of reflux, and the reaction was monitored by TLC until the raw materials disappeared completely, the reaction solution was spin-dried, and the reactant was redissolved in ethyl acetate, respectively With HCl (30mL, 3M) and NaHCO 3 (30mL, 3M) washed with water and extracted. The extract was collected and dried, spin-dried, and purified by column chromatography to obtain product 1a, a white solid, melting point: 165° C., yield 92%.
[0034] 1 H NMR (400MHz, CDCl 3 )δ8.11(d, J=3.8Hz, 1H), 7.96(dd, J=7.9, 1.4Hz, 1H), 7.84(d, J=7.9Hz, 1H), 7.46(t, J=...
Embodiment 2
[0036]Synthesis of 5-bromo-2-(3,4,5-trimethoxy-phenyl)-isoindole-1,3-dione 1b
[0037] Compound 4-bromophthalic anhydride (0.67mmol) and compound 3,4,5-trimethoxyaniline (0.80mmol) were separated according to the operation process of Example 1 to obtain compound 1b, a white solid, melting point: 200°C,
[0038] Yield 88%.
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.09(d, J=0.8Hz, 1H), 7.94(dd, J=7.9, 1.3Hz, 1H), 7.82(d, J=7.9Hz, 1H), 6.63(s, 2H), 3.89( s,3H),3.88(s,6H); ESI-MS m / z:392.1[M+H] + .
Embodiment 3
[0041] Synthesis of 6-(2,6-diisopropyl-phenyl)pyrrolo(3,4-b)pyridine-5,7-dione 1c
[0042] Compound 2,3-pyridinedicarboxylic acid anhydride (0.67mmol) and compound 2,6-diisopropylaniline (0.80mmol), according to the operation process of Example 1, were separated to obtain compound 1c, a white solid, melting point: 170 ° C, collected rate 56%.
[0043] 1 H NMR (400MHz, DMSO) δ9.11 (dd, J = 5.0, 1.4Hz, 1H), 8.46 (dd, J = 7.7, 1.4Hz, 1H), 7.92 (dd, J = 7.7Hz, 1H), 7.54 (t, J=7.7Hz, 1H), 7.37(d, J=7.7Hz, 2H), 2.73(hept, J=6.8Hz, 2H), 1.07(d, J=7.7Hz, 12H); ESI-MS m / z:309.2[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com