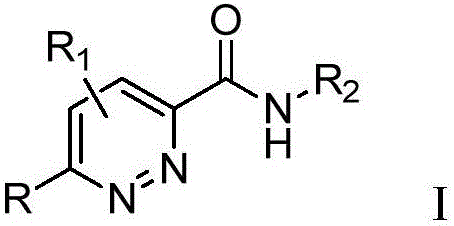
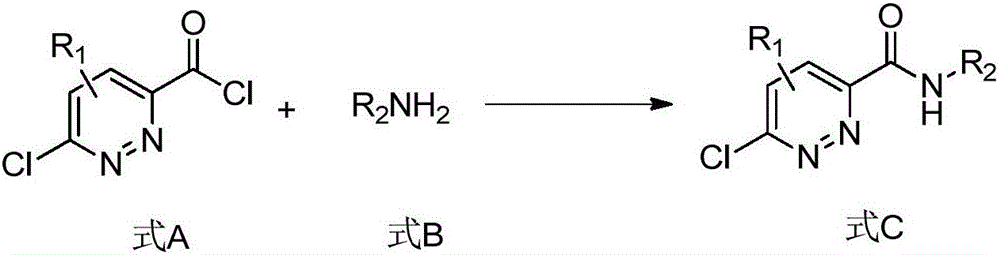Pyridazine derivative and preparation method and application thereof
A kind of derivative, pyridazine technology, applied in the field of medicine, can solve the problems such as unsatisfactory drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment 1: N-cyclohexyl-6-morpholine pyridazine-3-carboxamide (compound 1)
[0118] a) 1,4,5,6-tetrahydro-6-oxypyridazine-3-carboxylic acid (compound 1a)
[0119] Sodium hydroxide (0.69g, 17.25mmol) and hydrazine sulfate (1.02g, 7.85mmol) were placed in a 10mL double-necked flask, 4.5mL of hot water was added to dissolve it, and 1.8mL of hot α - Aqueous solution of ketoglutaric acid (1.14g, 7.81mmol), after the dropwise addition, the reaction solution was heated to slight boiling and reacted overnight. Stop the reaction, cool and stand in an ice bath to precipitate a white solid, which is suction filtered. The white solid obtained by suction filtration was recrystallized with 2N hydrochloric acid to obtain 691.1 mg of colorless needle crystals. Yield: 62%; Melting point: 197.6-200.3°C.
[0120] b) 1,6-dihydro-6-oxopyridazine-3-carboxylic acid (compound 1b)
[0121] Put compound 1a (568mg, 4.0mmol) in a 10mL double-necked flask, add 2mL of acetic acid, stir magnet...
Embodiment 2
[0130] Example 2: N-(adamantan-1-yl)-6-morpholine pyridazine-3-carboxamide (compound 2)
[0131] a) N-(adamantan-1-yl)-6-chloropyridazine-3-carboxamide (compound 2a)
[0132] The experimental method was the same as the preparation of compound 1d in Example 1, except that 1-adamantanamine was used instead of cyclohexylamine to obtain 103.6 mg of a yellow solid. Yield: 50%; Melting point: 200.8-202.1°C.
[0133] 1 H NMR (500MHz, CDCl 3 )δ8.25 (d, J=8.5Hz, 1H), 7.86 (s, 1H), 7.66 (d, J=8.5Hz, 1H), 2.16 (s, 9H), 1.78-1.72 (m, 6H).
[0134] b) N-(adamantan-1-yl)-6-morpholine pyridazine-3-carboxamide (compound 2)
[0135] The experimental method was the same as the preparation of compound 1 in Example 1 to obtain 19 mg of white solid. Yield: 93%; Melting point: 191.6-192°C.
[0136] 1 H NMR (500MHz, CDCl 3 )δ8.02(d, J=9.5Hz, 1H), 7.77(s, 1H), 6.96(d, J=9.5Hz, 1H), 3.86(t, J=4.5Hz, 4H), 3.72(t, J=5.0Hz, 4H), 2.14(s, 9H), 1.76-1.70(m, 6H).
Embodiment 3
[0137] Example 3: N-(adamantan-2-yl)-6-morpholine pyridazine-3-carboxamide (compound 3)
[0138] a) N-(adamantan-2-yl)-6-chloropyridazine-3-carboxamide (compound 3a)
[0139] The experimental method was the same as the preparation of compound 1d in Example 1, except that 2-adamantanamine was used instead of cyclohexylamine to obtain 105.7 mg of a yellow solid. Yield: 51%; Melting point: 171.2-172.5°C.
[0140] 1 H NMR (500MHz, CDCl 3 )δ8.46(d, J=5.0Hz, 1H), 8.29(d, J=8.5Hz, 1H), 7.69(d, J=8.5Hz, 1H), 4.30-4.28(m, 1H), 2.08( s, 2H), 1.97 (d, J = 13.5Hz, 2H), 1.92 (s, 6H), 1.80 (s, 2H), 1.72 (d, J = 13.0Hz, 2H).
[0141] b) N-(adamantan-2-yl)-6-morpholine pyridazine-3-carboxamide (compound 3)
[0142] The experimental method was the same as the preparation of compound 1 in Example 1 to obtain 19.4 mg of white solid. Yield: 85%; Melting point: 219.4-219.8°C.
[0143] 1 H NMR (500MHz, CDCl 3)δ8.34(d, J=8.0Hz, 1H), 8.05(d, J=9.5Hz, 1H), 6.97(d, J=9.5Hz, 1H), 4.27-4.25(m, J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com