DSD acid preparation method
A technology of mixing acid and sulfuric acid, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of low purity of finished products and high production labor intensity, and achieve the effects of high purity, short reaction time, and reduction of waste formation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
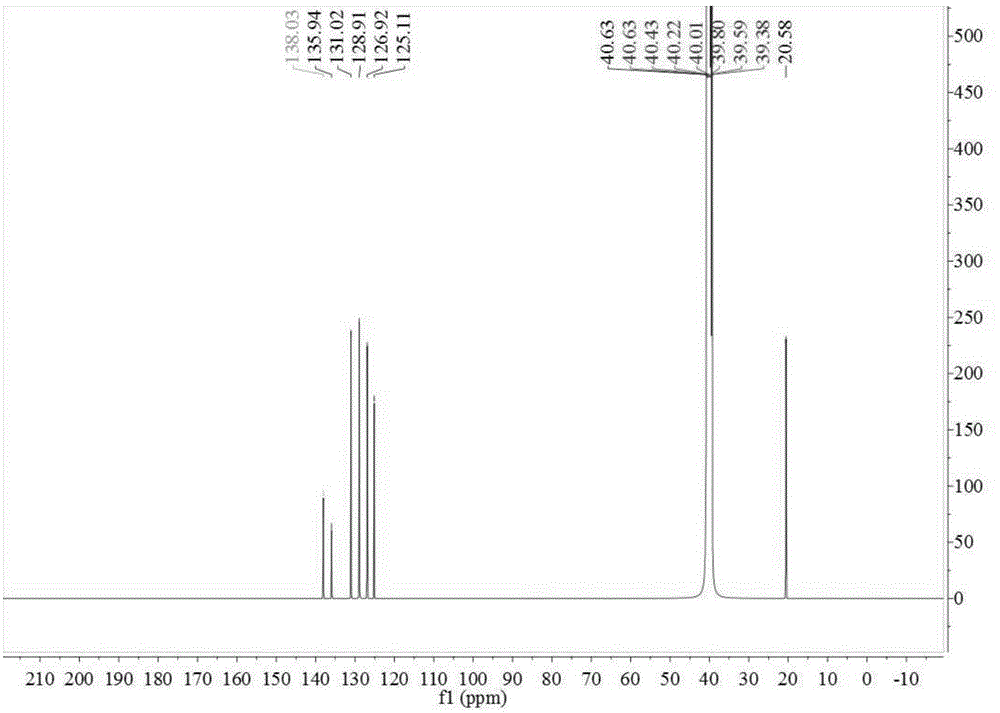
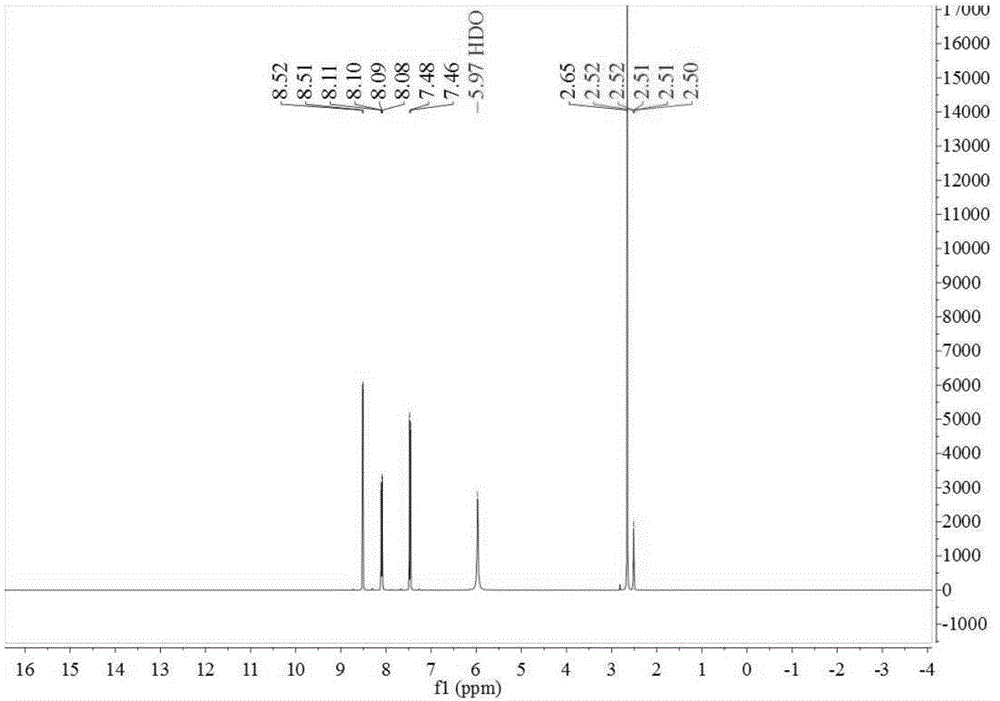
[0070] In the 500ml four-necked reaction flask, add 48.80g toluene, 0.30g catalytic fixer SO4 2- / ZrO 2 -TiO 2 , under electric stirring, the frozen brine is cooled to about -5~0°C, and the measured 98% concentrated sulfuric acid is slowly added dropwise, and the dropping time is controlled within 2~10 hours. until the reaction is complete. The reaction solution changed from cloudy to light yellow-green transparent liquid. Sample HPLC analysis, OTS 81.09%, PTS 17.65%. Add the reaction liquid to an appropriate amount of ice water, adjust the concentration of sulfuric acid to 75%, stir at a temperature of 10-15°C, keep stirring for 6 hours, and filter with suction to obtain a PTS filter cake (HPLC content 96.73%). The proton nuclear magnetic resonance spectrum of the prepared PTS is as follows figure 1 As shown, the carbon NMR spectrum is as figure 2 shown.
[0071] Carefully transfer the filtrate to another 500ml four-necked reaction bottle, adjust the acidity to 80%, c...
Embodiment 2
[0075] Add PNTS (100%) 56.7g synthesized in embodiment 1 in the 1L four-necked flask, 0.20g Cu-Fe-MnO x / TiO 2 , 600ml of water. A redox electrode and a potential measurement and control system are installed in the reaction bottle. Stir and heat up to 35°C, and slowly add 31.68% liquid caustic soda dropwise. When the temperature rises to 45°C, chlorine gas is introduced, and the reaction is exothermic. At this time, the temperature is gradually increased, and the flow rate and pressure of the chlorine gas are controlled so that the oxidation-reduction potential of the reaction system is between -100 and +100. As the reaction progressed, the reaction solution became turbid, and the color gradually changed from light yellow to light amber. After 1 hr, when the temperature rose to 75°C, the temperature was kept for 15 minutes, and the solution became clear, so the dropwise addition of liquid caustic soda was stopped. Liquid caustic soda consumes 170ml. HPLC analysis showed t...
Embodiment 3
[0077] In the 1L four-necked flask, add 56.7g of PNTS (100%) synthesized in Example 1, 0.20g Cu-Ce-MnO x / TiO 2, 500ml of water, redox electrode and potential measurement and control system are installed in the reaction bottle. Stir and heat up to 45° C., start to drop 170 ml of 31.68% liquid caustic soda, and feed chlorine gas when the pH value of the reaction solution is ≥10, and operate the potentiometric measurement and control system according to Example 2. When the temperature rose to 65°C, 150ml of water was added dropwise, and the addition was completed within half an hour. Finally, the temperature rose to 85°C and the solution became clear. HPLC analysis of PNTS content ≤ 1.0%, stop feeding chlorine, the reaction is over. Reaction time 105 minutes. Cooling crystallization and suction filtration, adding the waste acid produced in Example 2 dropwise to the mother liquor, adjusting the pH value to 3, and then suction filtration. The two filter cakes were evenly mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com