Composition containing maca and rhodiola rosea and preparation method and application thereof
A composition, the technology of Rhodiola rosea, is applied in the direction of medical preparations containing active ingredients, drug combinations, pharmaceutical formulas, etc., which can solve the problems of slow effect and not very obvious effect, so as to relieve physical fatigue, facilitate absorption, Immunity-boosting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
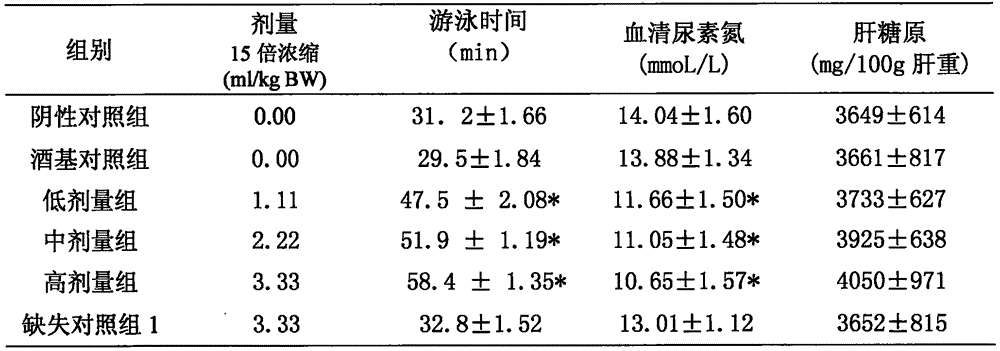
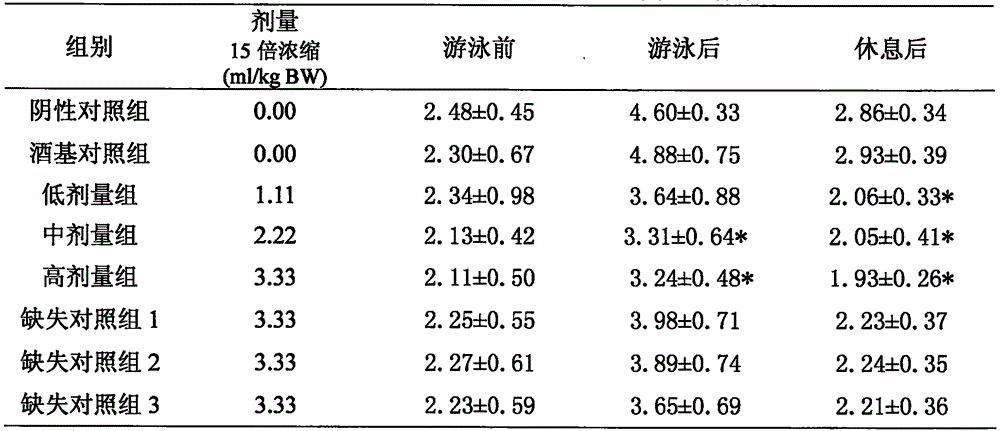
[0033] Ingredients: 80 parts of maca, 30 parts of rhodiola, 40 parts of American ginseng, 50 parts of Morinda officinalis, 60 parts of medlar
[0034] Preparation:
[0035] 1. Requirements for raw and auxiliary materials
[0036] Lycium barbarum, Morinda officinalis, American ginseng, and rhodiola decoction pieces meet the corresponding standard requirements of the "Pharmacopoeia of the People's Republic of China" (2010 edition); Maca uses pre-crushed maca powder; white wine (50%) meets the requirements of GB2757 Standard requirements; Purified water meets the requirements of Purified Water in Part Two of the Pharmacopoeia of the People's Republic of China (2010 Edition).
[0037] 2. Feeding and extraction
[0038] The raw materials of the decoction pieces are firstly selected, and the decoction pieces of maca powder, medlar, Morinda officinalis, American ginseng, and rhodiola are taken according to the formula. In the extraction tank, add 20 times the amount of white wine ...
Embodiment 2
[0048] Components: 60 parts of maca, 15 parts of rhodiola, 25 parts of American ginseng, 30 parts of Morinda officinalis, 50 parts of medlar
[0049] Preparation:
[0050] 1. Requirements for raw and auxiliary materials
[0051] Lycium barbarum, Morinda officinalis, American ginseng, and rhodiola decoction pieces meet the corresponding standard requirements of the "Pharmacopoeia of the People's Republic of China" (2010 edition); Maca uses pre-crushed maca powder; white wine (50%) meets the requirements of GB2757 Standard requirements; Purified water meets the requirements of Purified Water in Part Two of the Pharmacopoeia of the People's Republic of China (2010 Edition).
[0052] 2. Feeding and extraction
[0053] The raw materials of the decoction pieces are firstly selected, and the decoction pieces of maca powder, medlar, Morinda officinalis, American ginseng, and rhodiola are taken according to the formula. In the extraction tank, add 15 times the amount of white wine (...
Embodiment 3
[0063] Ingredients: 40 parts of maca, 10 parts of rhodiola, 15 parts of American ginseng, 20 parts of Morinda officinalis, 30 parts of medlar
[0064] Preparation:
[0065] 1. Requirements for raw and auxiliary materials
[0066] Lycium barbarum, Morinda officinalis, American ginseng, and rhodiola decoction pieces meet the corresponding standard requirements of the "Pharmacopoeia of the People's Republic of China" (2010 edition); Maca uses pre-crushed maca powder; white wine (50%) meets the requirements of GB2757 Standard requirements; Purified water meets the requirements of Purified Water in Part Two of the Pharmacopoeia of the People's Republic of China (2010 Edition).
[0067] 2. Feeding and extraction
[0068] The raw materials of the decoction pieces are firstly selected, and the decoction pieces of maca powder, medlar, Morinda officinalis, American ginseng, and rhodiola are taken according to the formula. In the extraction tank, add 10 times the amount of white wine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com