Lithium reagent composition, lithium ion measurement method and measurement device using the composition
A determination method and composition technology, which can be used in measurement devices, chemical method analysis, and analysis using chemical indicators, etc., and can solve problems such as unavailability, high price, and unstable supply of original medicines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
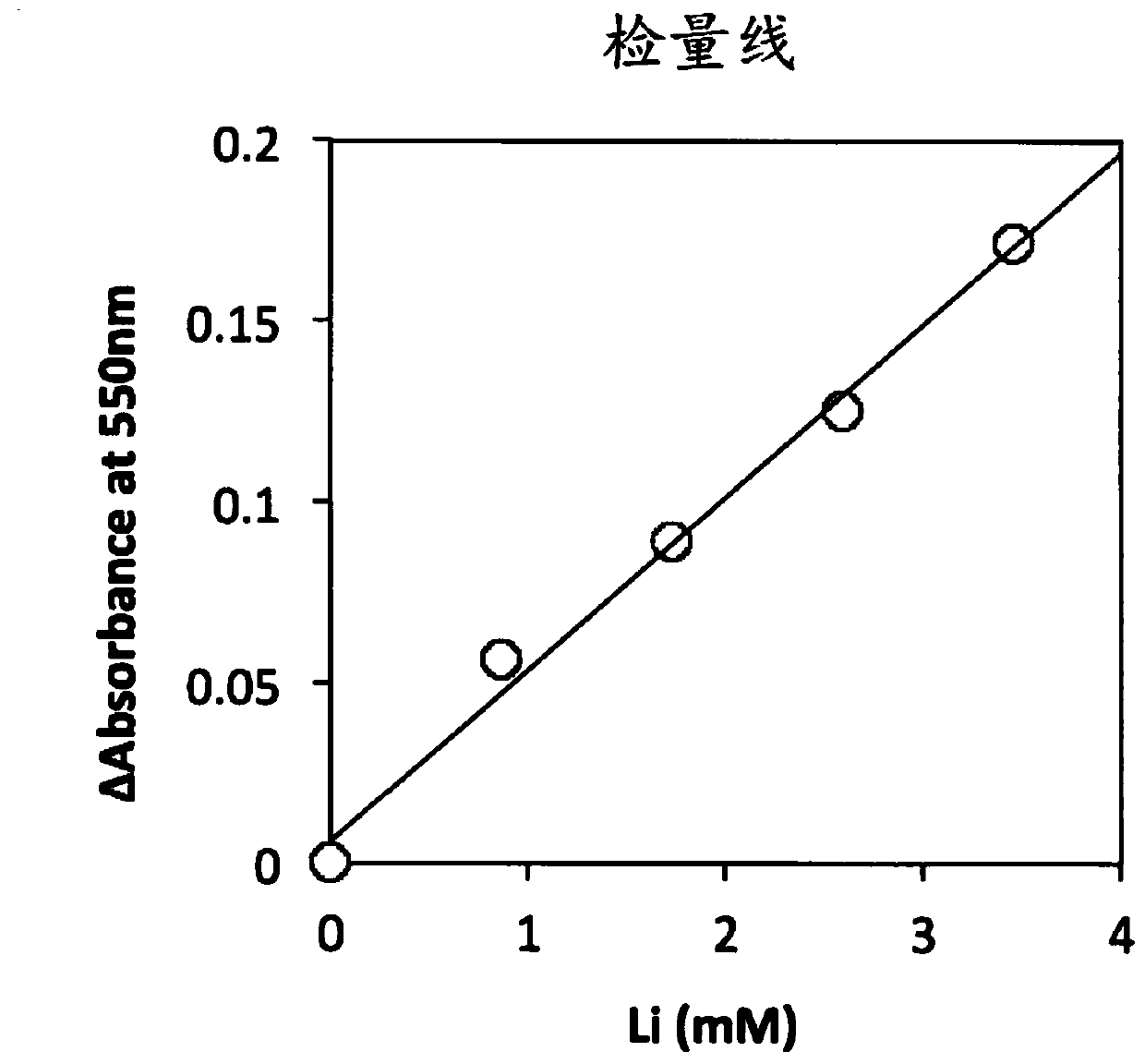
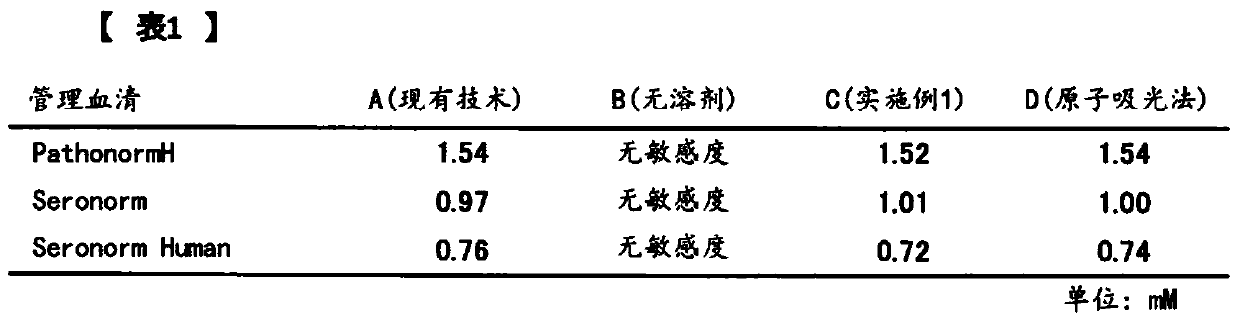
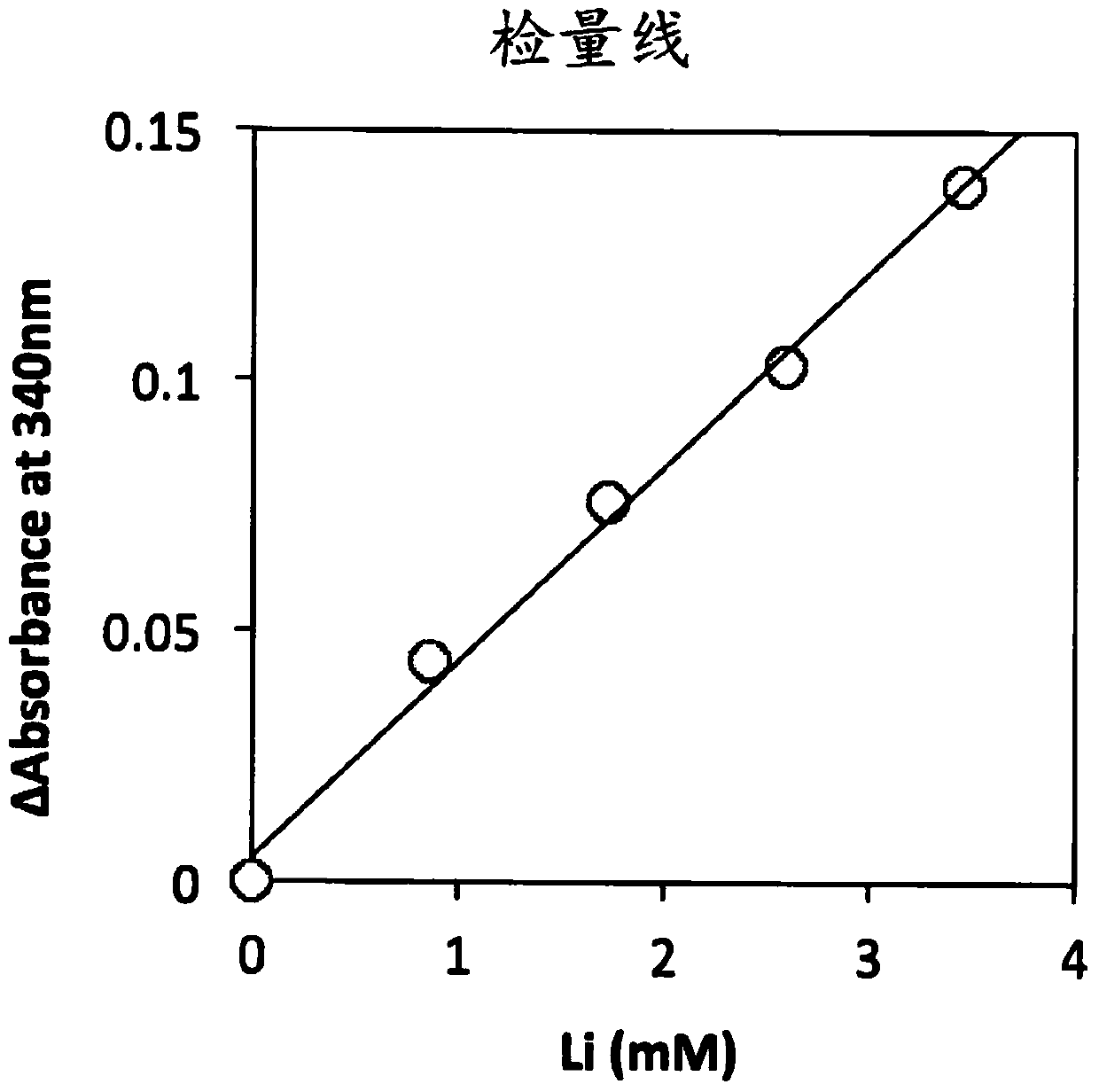
Image
Examples
Embodiment 1
[0093] Embodiment 1 (sample 1)
[0094] The composition of the lithium reagent as Example 1 (reagent 1) is as follows.
[0095] Chelating agent: F28 tetraphenylporphyrin 0.01% by weight
[0096] Multifunctional conditioner: triethanolamine 1% by weight
[0097] Stabilizer (nonionic surfactant):
[0098] Triton X-100 (registered trademark)
[0099] (Polyoxyethylene octylphenyl ether) 1% by weight
[0100] Stabilizer (anionic surfactant):
[0101] Sodium Lauryl Sulfate 1% by weight
[0102] Masking agent: EDTA-2K 0.04% by weight
[0103] As indicated above, this reagent does not contain organic solvents. Moreover, as a substance described as a multifunctional regulator, the triethanolamine in Example 1 has the functions of a dispersant, a buffer, an emulsifier, a complexing agent, etc., and it is also considered in Example 1 that it can be used as any regulator. agent works.
[0104] Sodium hydroxide was added to the above reagent to adjust the pH to 10.8, and purified ...
Embodiment 2
[0142] Embodiment 2 (sample 2)
[0143] Next, to illustrate Example 2, the composition of Example 2 and Example 1 is different, mainly because the consumption of triethanolamine has been increased, and the masking agent has changed somewhat, and the lithium reagent of Example 2 (reagent 2) of the following composition is made , verify the composition of this embodiment 2.
[0144] The composition of the lithium reagent of Example 2 (reagent 2) is as follows.
[0145] Chelating agent: F28 tetraphenylporphyrin 0.01% by weight
[0146] Multifunctional conditioner: triethanolamine 3.7% by weight
[0147] Stabilizer (nonionic surfactant): Triton-100 (registered trademark)
[0148] (Polyoxyethylene octylphenyl ether) 1.5% by weight
[0149] Stabilizer (anionic surfactant):
[0150] Sodium Lauryl Sulfate 1% by weight
[0151] Masking agent: EDTA-2K 0.045% by weight
[0152] As mentioned above, no organic solvent is contained in this reagent.
[0153] Sodium hydroxide was adde...
Embodiment 3
[0160] Embodiment 3 (sample 3)
[0161] Next, Example 3 will be described. The composition of Example 3 is different from that of Example 1. Diethanolamine was used instead of triethanolamine to prepare the lithium reagent of Example 3 (Reagent 3) with the following composition. 2 for verification.
[0162] The composition of the lithium reagent of Example 3 (Reagent 3) is as follows.
[0163] Chelating agent: F28 tetraphenylporphyrin 0.01% by weight
[0164] Multifunctional conditioner: diethanolamine 3.7% by weight
[0165] Stabilizer (nonionic surfactant):
[0166] Triton-100 (registered trademark)
[0167] (Polyoxyethylene octylphenyl ether) 1% by weight
[0168] Stabilizer (anionic surfactant):
[0169] Sodium Lauryl Sulfate 1% by weight
[0170] Masking agent: EDTA-2K 0.04% by weight
[0171] As mentioned above, this reagent does not contain organic solvents. Moreover, in Example 3, diethanolamine is described as a multifunctional regulator, which also has the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com