Quantum dot and immunochromatography test strip rapid detection method for beta-stimulant multiresidue
A technology of immunochromatography and detection method, which is applied in the field of rapid detection of quantum dot immunochromatographic test strips with multi-residue β-agonists, can solve the problem of quantitative analysis of samples that are difficult to detect, and the inability to screen multiple β-agonists. and other problems, to achieve the effect of wide excitation wavelength range, convenient operation and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Quantum dot immunochromatographic test strip rapid detection method with multiple residues of β-agonists
[0073] A method for rapid detection of quantum dot immunochromatographic test strips with multiple residues of β-agonists, comprising the following steps:
[0074] (1) Preparation of quantum dot fluorescent probe: Dissolve 20 μL of quantum dots in 3.2 mL of 0.01mol / L pH7.4 phosphate buffer, add coupling agent 1-(3-dimethylpropyl)-3- Ethylcarbodiimide hydrochloride, N-hydroxysuccinimide solution, stirred at room temperature for 15 minutes, then added 50 μg β-agonist monoclonal antibody, stirred at room temperature for 1.5 hours, continued to add 0.4 mL of 10% BSA solution to block After reacting for 1 hour, centrifuge the reaction solution at 12,000 r / min for 40 minutes, remove the supernatant, redissolve the precipitate with reconstitution solution, and obtain the labeled complex of quantum dots and β-agonist monoclonal antibody, and store in the dark at ...
Embodiment 2
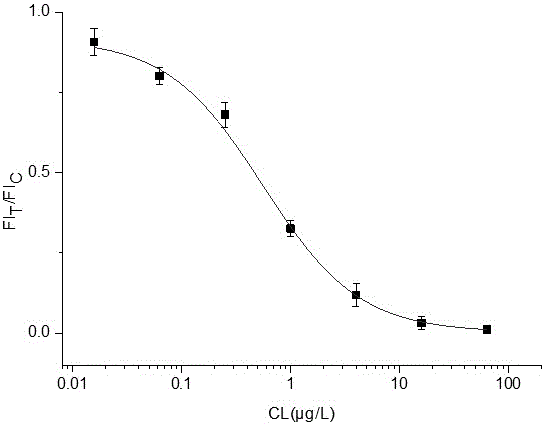
[0101] Example 2 Taking the standard clenbuterol hydrochloride (CL) as an example to establish a standard curve for quantum dot immunochromatography test strips
[0102] 1. Experimental materials
[0103] (1) β-agonist broad-spectrum monoclonal antibody, the preparation method is the same as in Example 1.
[0104](2) Goat anti-mouse IgG secondary antibody is a commercial reagent, purchased from Guangzhou Youkangduo Biotechnology Co., Ltd.
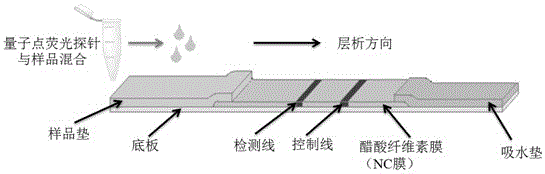
[0105] The preparation of the immunochromatographic test strip is the same as in Example 1.
[0106] 2. Configuration of CL standard solution, using Tris-HCl to prepare a series of standard solutions with concentrations of 0, 0.0156, 0.0625, 0.250, 1.00, 4.00, 16.00 and 64.0 μg / L, respectively, for test strip detection.
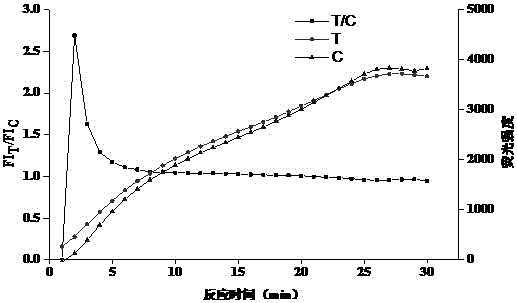
[0107] 3. Determination of test strips
[0108] Each concentration was tested 3 times, and after 10 min, the test strip FI was read with a fluorescent immunochromatography reader. T / FI C ratio. to FI T / FI C is th...
Embodiment 3
[0110] The precision of embodiment 3 quantum dot immunochromatography test strips
[0111] 1. Taking CL as an example, use different batches of test strips to test standard solutions with different CL contents, repeat the detection 3 times for each concentration, measure 3 different batches, and calculate the actual detection value.
[0112] 2. As shown in Table 1, the inter-assay and intra-assay coefficients of variation of test strip test results are both less than 15%, indicating that the method has good repeatability.
[0113] Table 1 Intra-assay and inter-assay variation of quantum dot immunochromatographic test strip detection methods
[0114]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com