HRM method and kit for clinically detecting deafness-related gene mutation
A kit and gene technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of high reagent consumption, low sensitivity, and deafness-related gene mutation detection methods that cannot meet clinical needs. The effect of strong specificity and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Deafness-related gene mutation detection of artificially synthesized DNA quality control product
[0070] (1) Diluted DNA quality control
[0071] The DNA quality control product was DNA with different mutation contents at five mutation sites of deafness-related genes synthesized by Shanghai Sangon Bioengineering Co., Ltd. Dilute the DNA quality control substance to 0.1ng / μL with sterile double distilled water.
[0072] (2) The primer sequences and detected mutation sites are shown in Table 2.
[0073] Table 2. Primer sequences and detection site information
[0074]
[0075]
[0076] The 5 pairs of primers in Table 2 were used for PCR reaction to amplify the target DNA. The PCR reaction solution included 10×buffer (Biostar), 10 μmol / L upstream and downstream primers, 10mmol / L dNTPs (Roche), 2U Taq DNA polymerase (Biostar), LC GREEN (IdahoTechnologyInc, USA) and sterile water.
[0077] 10 μL PCR reaction system: Taq buffer (10×) 1 μL, dNTPs (10 mM)...
Embodiment 2
[0083] The detection of embodiment 2 clinical specimens
[0084] (1) Extract the DNA template from the patient's peripheral blood
[0085] ①Take 200 μL of anticoagulated blood, ②Use the TIANamp Genomic DNA Kit (TIANGEN Company, China) to extract DNA from the whole blood sample, and operate according to the instructions.
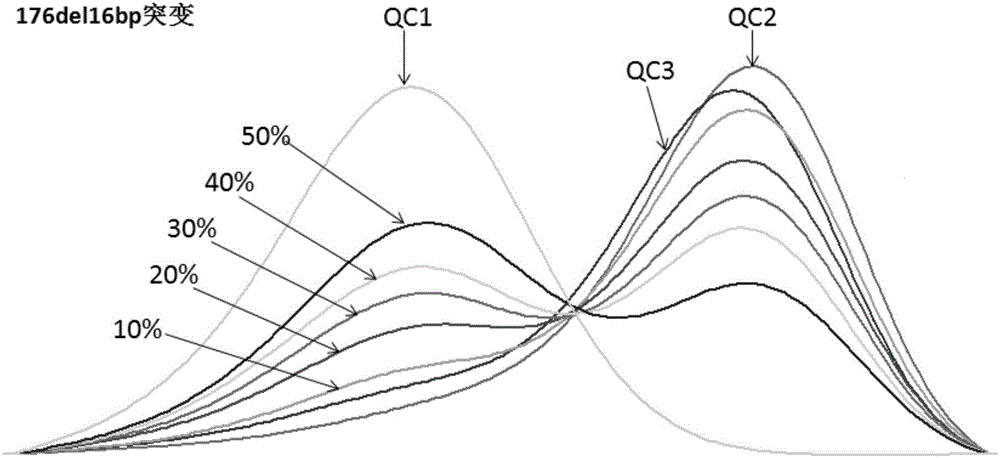
[0086] (2) According to the method of Example 1, the double-blind detection of deafness gene mutation was carried out to 30 cases of clinical deafness patient specimens (Wuhan City Women and Children's Health Care Center, Wuhan, Hubei) and the quality control product synthesized by Shanghai Sangon Bioengineering Co., Ltd. see results Figure 6-10 and Table 3-7. The test results were in good agreement with the clinical report results (sequencing method).
[0087] Table 3. Comparison of detection results of clinical specimens with 176del16bp mutation in the coding region of GJB2 gene
[0088] Sample serial number
HRM method
Sequencing
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com