Preparation method of 2-hydroxymethyl-4-(methoxypropoxy)-3-methylpyridine hydrochloride
A technology of methylpyridine hydrochloride and methoxypropoxy, which is applied in the field of preparation of 2-hydroxymethyl-4--3-methylpyridine hydrochloride, can solve the problem that wastewater treatment is difficult and has not been mentioned. And, it is not suitable for industrial application, etc., to achieve the effect of good solid, improved purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
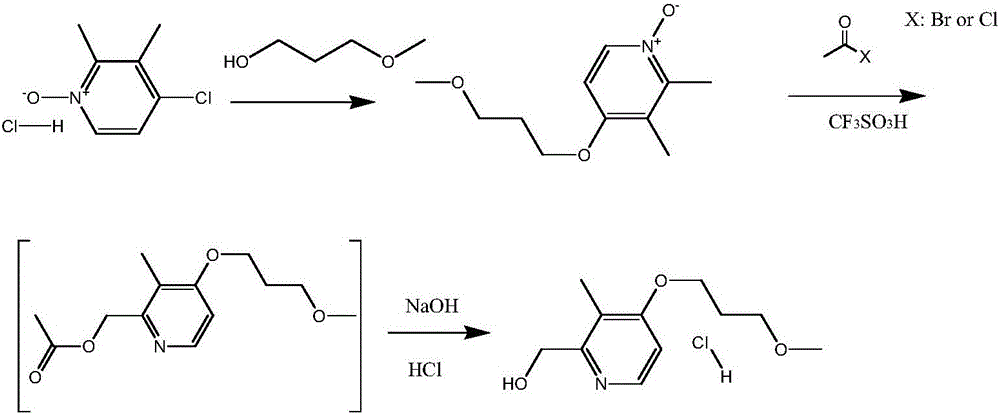
[0027] Add 68g of 3-methoxypropanol into the reaction flask, add 90g of powdered sodium hydroxide under stirring, and react at 60-70°C for 30 minutes after the addition is complete. Then 2.5 g of tetrabutylammonium bromide and 105 g of 2,3-dimethyl-4-chloropyridine-N-oxide hydrochloride were added, and the stirring reaction was continued at 90-95°C for 6 hours. Cool down to room temperature, add 500ml of dichloromethane and 500ml of water, separate the phases, extract the water phase with dichloromethane three times, combine the organic phases, recover the dichloromethane, and obtain about 115g of a reddish-brown residue, which is 2,3-dichloromethane. Methyl-4-(methoxypropoxy)-pyridine-N-oxide was directly used in the next reaction.
[0028] Mix 110g of 2,3-dimethyl-4-(methoxypropoxy)-pyridine-N-oxide with 12g of trifluoromethanesulfonic acid, slowly add 64g of acetyl chloride dropwise at room temperature, after the drop , keep warm at 15-35°C for 5 hours; slowly add 185ml of...
Embodiment 2
[0030] Referring to Example 1, 2,3-dimethyl-4-(methoxypropoxy)-pyridine-N-oxide was prepared.
[0031]Mix 110g of 2,3-dimethyl-4-(methoxypropoxy)-pyridine-N-oxide with 12g of trifluoromethanesulfonic acid, slowly add 64g of acetyl chloride dropwise at room temperature, after the drop , keep warm at 15-35°C for 5 hours; slowly add 185ml of water after the warm-keeping reaction, adjust the pH of the system to 13-14 with sodium hydroxide solution, then raise the temperature to 60-65°C and keep warm for 5 hours; keep warm After finishing, cool to room temperature, add 340ml of toluene, separate the phases, extract the water phase with toluene twice, combine the toluene phases, and concentrate to dryness under reduced pressure to obtain about 87g of the concentrate, which is dissolved in 150ml of ethyl acetate and cooled to below 5°C , then use saturated HCl ethyl acetate solution to adjust pH=3~4, stir and crystallize below 5°C for about 2 hours; filter with suction, wash the filt...
Embodiment 3
[0033] Referring to Example 1, 2,3-dimethyl-4-(methoxypropoxy)-pyridine-N-oxide was prepared.
[0034] Mix 110g of 2,3-dimethyl-4-(methoxypropoxy)-pyridine-N-oxide with 12g of trifluoromethanesulfonic acid, slowly add 64g of acetyl chloride dropwise at room temperature, after the drop , keep warm at 15-35°C for 5 hours; slowly add 185ml of water after the warm-keeping reaction, adjust the pH of the system to 13-14 with sodium hydroxide solution, then raise the temperature to 60-65°C and keep warm for 5 hours; keep warm After finishing, cool to room temperature, add 340ml of toluene, separate the phases, extract the water phase with toluene twice, combine the toluene phases, dry and dehydrate with anhydrous sodium sulfate, cool to below 5°C, and adjust the pH with saturated HCl ethyl acetate solution = 3 to 4, stirred and crystallized at below 5°C for about 2 hours; filtered with suction, washed the filter cake with cold acetone, and dried under reduced pressure at about 40°C t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com