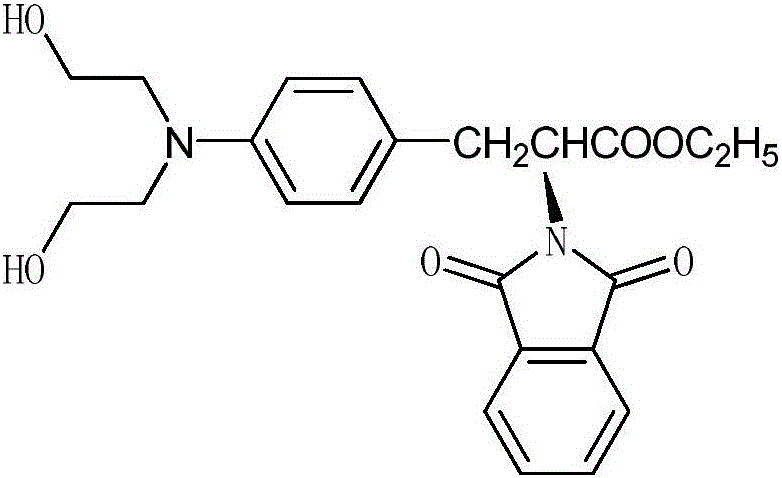
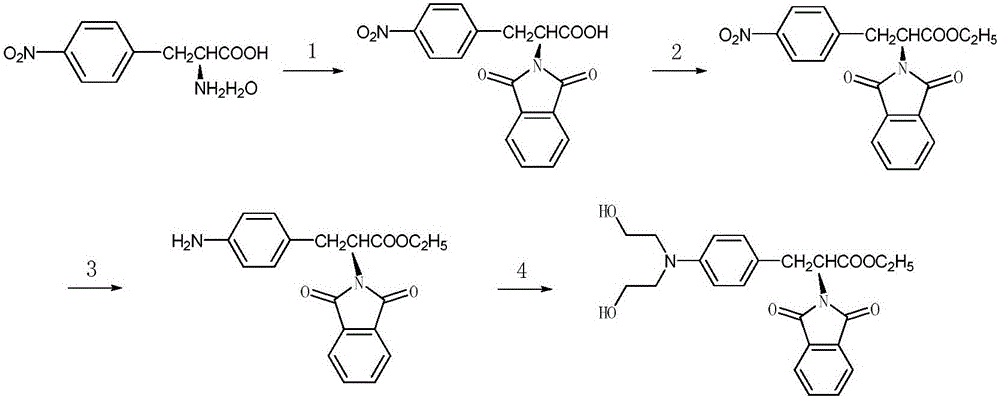
Synthesis process of N-paraphthalyl(diethanol)amino-L-phenylalanine ethyl ester
A technology of phthaloyl p-phenylalanine ethyl ester, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of long synthetic route, low yield, high toxicity, etc., and achieve high overall yield and high safety , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of synthetic technique of N-phthaloyl p-(dihydroxyethyl) amino-L-phenylalanine ethyl ester, the concrete steps of described technique are as follows:
[0028] (1) Amino protection reaction:
[0029] In a three-necked flask, add 120 grams of p-nitro-L-phenylalanine hydrate, 500 milliliters of toluene and 100 milliliters of triethylamine to mix, add 85 grams of phthalic anhydride under stirring, heat to reflux, and react 3 Hour, after reaction finishes, be cooled to room temperature, the reaction solution that obtains is poured in the hydrochloric acid solution, after fully stirring, filter and obtain 158 gram N-phthaloyl p-nitro-L-phenylalanine solids, the step The yield is 90%.
[0030] (2) Esterification reaction:
[0031] Get 100 grams of N-phthaloyl-p-nitro-L-phenylalanine and 500 milliliters of ethanol and mix them in a three-necked flask, add 80 grams of thionyl chloride dropwise while stirring, and heat to Reflux, react for 3 hours, cool to room temperat...
Embodiment 2
[0038] The synthetic technique of N-phthaloyl p-(dihydroxyethyl) amino-L-phenylalanine ethyl ester, described technique comprises the following steps:
[0039] (1) Amino protection reaction: dissolve the hydrate of raw material p-nitro-L-phenylalanine with solvent and triethylamine, add phthalic anhydride in the obtained raw material mixture, heat and reflux for reaction, and the obtained reaction The liquid is poured into the hydrochloric acid solution, and after fully stirring, N-phthaloyl-p-nitro-L-phenylalanine is obtained;
[0040] (2) Esterification reaction: dissolve the product obtained in step 1 with ethanol, add a chlorinating agent to the obtained mixed solution, and heat to reflux for reaction to obtain N-phthaloyl-p-nitro-L-phenylalanine ethyl ester;
[0041] (3) Reduction reaction: Dissolve the product obtained in step 2 with a solvent, place the obtained mixed solution in a reactor, add a catalyst to the reactor, and feed hydrogen gas under certain conditions t...
Embodiment 3
[0053] The synthetic technique of N-phthaloyl p-(dihydroxyethyl) amino-L-phenylalanine ethyl ester, described technique comprises the following steps:
[0054] (1) Amino protection reaction: dissolve the hydrate of raw material p-nitro-L-phenylalanine with solvent and triethylamine, add phthalic anhydride in the obtained raw material mixture, heat and reflux for reaction, and the obtained reaction The liquid is poured into the hydrochloric acid solution, and after fully stirring, N-phthaloyl-p-nitro-L-phenylalanine is obtained;
[0055] (2) Esterification reaction: dissolve the product obtained in step 1 with ethanol, add a chlorinating agent to the obtained mixed solution, and heat to reflux for reaction to obtain N-phthaloyl-p-nitro-L-phenylalanine ethyl ester;
[0056] (3) Reduction reaction: dissolve the product obtained in step 2 with a solvent, place the obtained mixed solution in a reactor, add a catalyst to the reactor, and feed hydrogen gas under certain conditions t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com