Production method for ferrum-free Zr(SO4)2
A production method and SO4 technology, applied in zirconium sulfate and other directions, can solve the problems of serious equipment corrosion, incomplete method, difficult filtration, etc., and achieve the effects of small product loss, complete separation and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] a. The zirconium sulfate solution containing ferrous ions and ferric ions is placed in a container, continuously stirred and added dropwise with a mass fraction of 15% hydroxylamine hydrochloride solution in the container, when thiocyanate is added dropwise to the zirconium sulfate solution When the potassium acid potassium solution is no longer red, it is considered that ferric ions are no longer detected; then the pH value of the solution is adjusted to 4 with a mass fraction of 26% ammonia solution; during this process, the following chemical reaction equation occurs:
[0022] 2Fe 3+ +2NH 2 OH·HCl=2Fe 2+ +N 2 ↑+4H + +2H 2 O+2Cl -
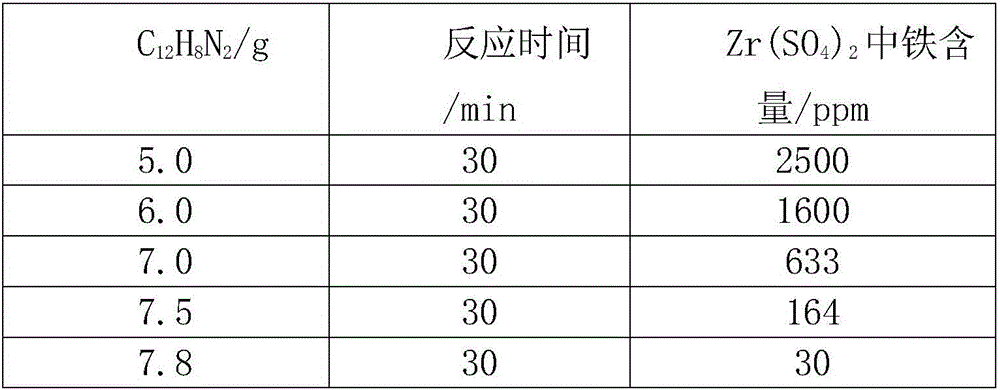
[0023] b. Stir continuously and add dropwise an o-phenanthroline solution with a mass fraction of 8% to the solution reacted in step a, and add potassium ferricyanide dropwise to the solution. When no blue precipitate is generated, it is deemed unsafe Ferrous ions were detected again. Continuously dripping the sodium chloride solut...
Embodiment 2
[0028] a. The zirconium sulfate solution containing ferrous ions and ferric ions is placed in a container, continuously stirred and added dropwise with a mass fraction of 10% hydroxylamine hydrochloride solution in the container, when adding thiocyanate dropwise to the zirconium sulfate solution When the potassium acid potassium solution is no longer red, it is considered that there is no ferric ion detected; then the pH value of the solution is adjusted to 5 with a mass fraction of 25% ammonia solution;
[0029] b. Stir constantly and add dropwise the o-phenanthroline solution with a mass fraction of 5% in the solution reacted in step a, and add potassium ferricyanide dropwise in the solution. Ferrous ions were detected again. Continuously add the calcium chloride solution that mass fraction is 30% in the container again, until there is no precipitation to generate again, after filtering, the liquid of gained is iron-free Zr(SO 4 ) 2 solution;
[0030] c. Put the solid rea...
Embodiment 3
[0032] a. The zirconium sulfate solution containing ferrous ions and ferric ions is placed in a container, continuously stirred and added dropwise with a mass fraction of 20% hydroxylamine hydrochloride solution in the container, when adding thiocyanate dropwise to the zirconium sulfate solution When the potassium acid potassium solution is no longer red, it is considered that ferric ions are no longer detected; then the pH value of the solution is adjusted to 3 with a mass fraction of 28% ammonia solution;
[0033] b. Stir continuously and add dropwise the o-phenanthroline solution with a mass fraction of 10% in the solution reacted in step a, and add potassium ferricyanide dropwise in the solution. Ferrous ions were detected again. Continuously dripping the potassium chloride solution that the mass fraction is 40% in the container again, until there is no precipitation to generate again, after filtering, the gained liquid is iron-free Zr(SO 4 ) 2 solution;
[0034] c. Put...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com