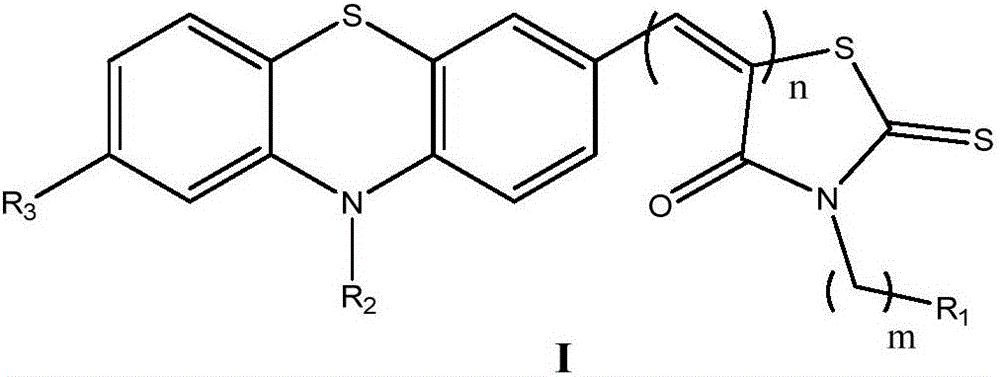
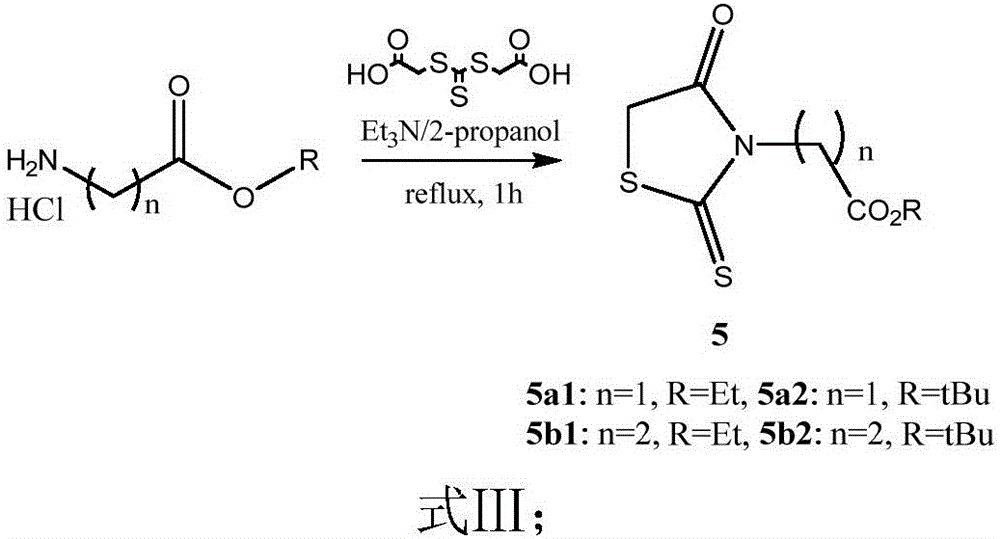
Novel phenothiazine derivative and preparation method and application thereof
A phenothiazine derivative, phenothiazine technology, applied in the field of biomedicine, to achieve good inhibitory effect and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
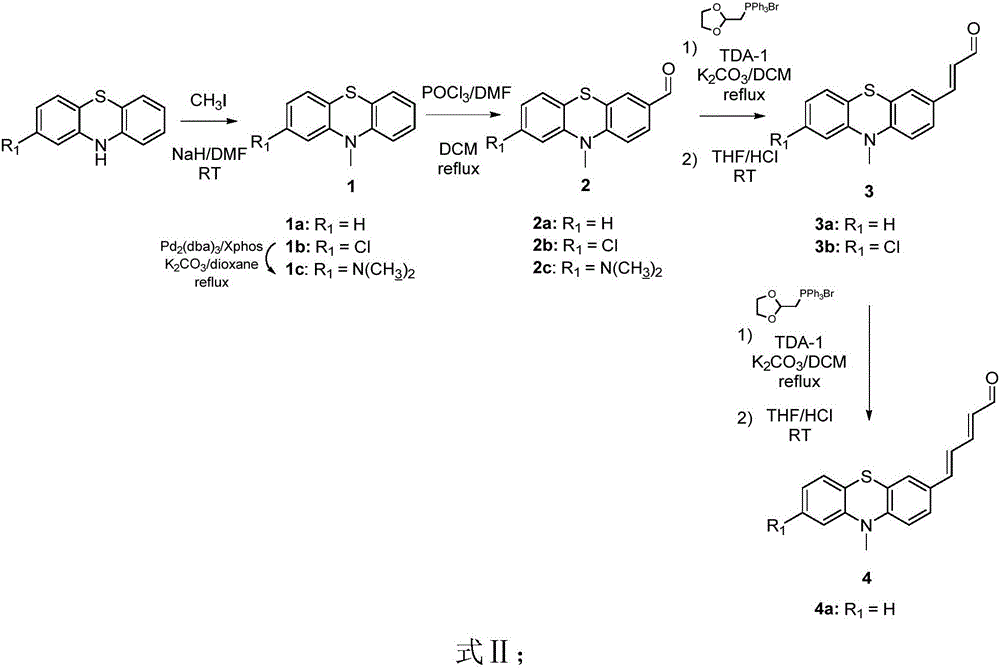
[0039] Embodiment 1: synthetic 10-methyl-10H-phenothiazine-3-formaldehyde 2a
[0040] Weigh NaH (60% purity, 1.0g, 25.1mmol) and slowly add it into a 50mL round bottom bottle with DMF (10mL) in an ice-water bath, then add methyl iodide (1.3g, 11.04mmol) and compound phenothiazine (2g , 10.0mmol), transferred to room temperature and stirred for 2h. After the reaction was monitored by TLC, water was added, extracted with DCM (50mL x 3), dried over magnesium sulfate and then concentrated to dryness. The product 1a (2.1g) was obtained by silica gel column separation as a white solid , melting point: 96°C, yield 97%.
[0041] 1 H NMR (400MHz, (CD 3 ) 2 CO)δ7.21(td, J=8.0,1.5Hz,2H),7.21(d,J=2.0Hz,1H),7.14(d,J=1.5Hz,1H),6.96-6.93(m,4H) ,3.39(s,3H).
[0042] In an ice-water bath at 0°C, slowly drop phosphorus oxychloride (1.98g, 1.18mL, 12.9mmol) into a 25mL round-bottom bottle with dry DMF (904L, 11.73mmol), stir for 0.5h, then add Compound 1a (500 mg, 2.34 mmol) in 5 mL DCM, t...
Embodiment 2
[0044] Embodiment 2: synthetic 8-chloro-10-methyl-10H-phenothiazine-3-formaldehyde 2b
[0045] Referring to the first step of the operation process in Example 1, the product 1b was isolated as a white solid, melting point: 75°C, yield 97%;
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.16(td, J=7.8,1.3Hz,1H),7.12(dd,J=7.8,1.3Hz,1H),6.99(d,J=8.5Hz,1H),6.94(t,J=7.5 Hz,1H),6.88(dd,J=8.5,2.0Hz,1H),6.78(d,J=8.5Hz,1H),6.73(d,J=2.0Hz,1H),3.29(s,3H).
[0047] Referring to the second step operation process of Example 1, the product 2b was isolated as a yellow solid, melting point: 160° C., yield 38%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ7.16(td, J=7.8,1.4Hz,1H),7.11(dd,J=7.8,1.4Hz,1H),7.01(d,J=8.2Hz,1H),6.93(td,J=7.6 ,1.4Hz,1H),6.88(dd,J=8.2,2.0Hz,1H),6.8(d,J=8.2Hz,1H),6.75(d,J=2.0Hz,1H),3.35(s,3H ).
Embodiment 3
[0049] Embodiment 3: synthetic 8-(dimethylamino)-10-methyl-10H-phenothiazine-3-formaldehyde 2c
[0050] Dimethylamine (1.6mL, 3.1mmol, 1.5eq) was added to dioxane (4mL) dissolved in compound 1b (500mg, 2.02mmol, 1eq) followed by the catalyst tris(dibenzylideneacetone)dipalladium (75mg, 0.08mmol, 0.04eq) and the ligand 2-dicyclohexylphosphonium-2,4,6-triisopropylbiphenyl (78mg, 0.16eq, 0.08eq) and cesium carbonate (1.4g, 2eq), Under the protection of argon, the reaction was carried out at 120°C for 7h. After the reaction was monitored by TLC, suction filtration was performed after cooling to room temperature. After the obtained filtrate was concentrated to dryness, the product 1c (260 mg) was separated by column chromatography, a white liquid, and the yield was 50%.
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.16-7.10 (m, 2H), 6.97 (d, J = 8.5, 1H), 6.89 (td, J = 8.0, 1.0Hz, 1H), 6.8 (dd, J = 8.0, 1.0Hz, 1H) ,6.33(dd,J=8.0,2.5Hz,1H),6.21(d,J=2.5,1H),3.36(s,3H),2.92(s,6H).
[0052]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com