Method for measuring dissolution rates of esomeprazole magnesium enteric-coated preparation in different media
A technology for esomeprazole magnesium and enteric-coated preparations, which is applied in the field of pharmaceutical preparation analysis and detection, can solve the problem of accurate calculation of unfavorable dissolution results, and difficulty in accurately evaluating the dissolution process of esomeprazole magnesium enteric-coated preparations. Discover research reports and other issues to achieve the effect of strong practicability, high sensitivity and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
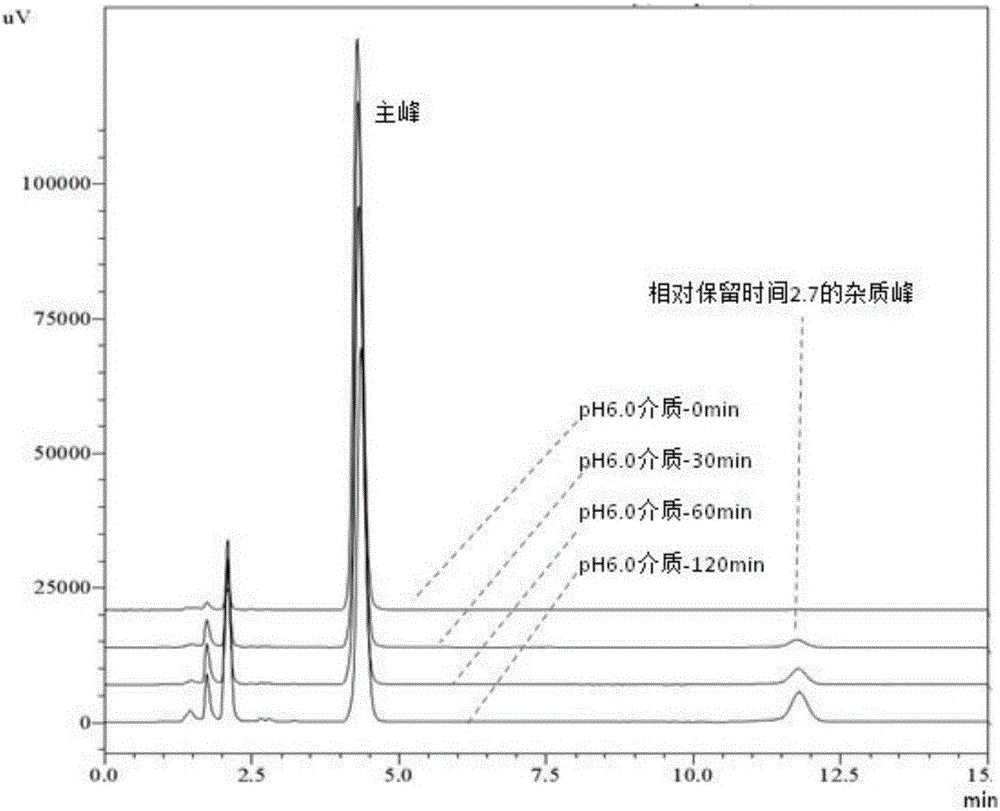
[0041] Example 1 Degradation Law of Esomeprazole Magnesium Enteric-coated Tablets or Enteric-coated Pellet Capsules in Phosphate Buffered Saline at pH 6.0
[0042] Take the specification of 20mg Esomeprazole magnesium enteric-coated tablets or enteric-coated micropill capsules (remove the capsule shell, take the contents, the same below), put in a mortar, grind finely, weigh 100mg into a 20ml volumetric flask, add 0.0125mol / L Sonicate 12mL of sodium hydroxide solution for 5min, add 7.5ml of ethanol to continue sonicating for 3min, and dilute to volume with 0.0125mol / L sodium hydroxide solution. Filtrate, take 2ml of the filtrate into a 100ml volumetric flask, make up to volume with pH6.0 phosphate buffer, put it in a water bath at 37°C±0.5°C, and set at 0, 15, 30, 45, 60, 90, 120, 180, 240min respectively Take a sample, filter. Take 5ml of the continued filtrate into a vial, add 600μl of 0.5mol / L sodium hydroxide respectively, mix well, accurately draw 20μl into the liquid ...
Embodiment 2
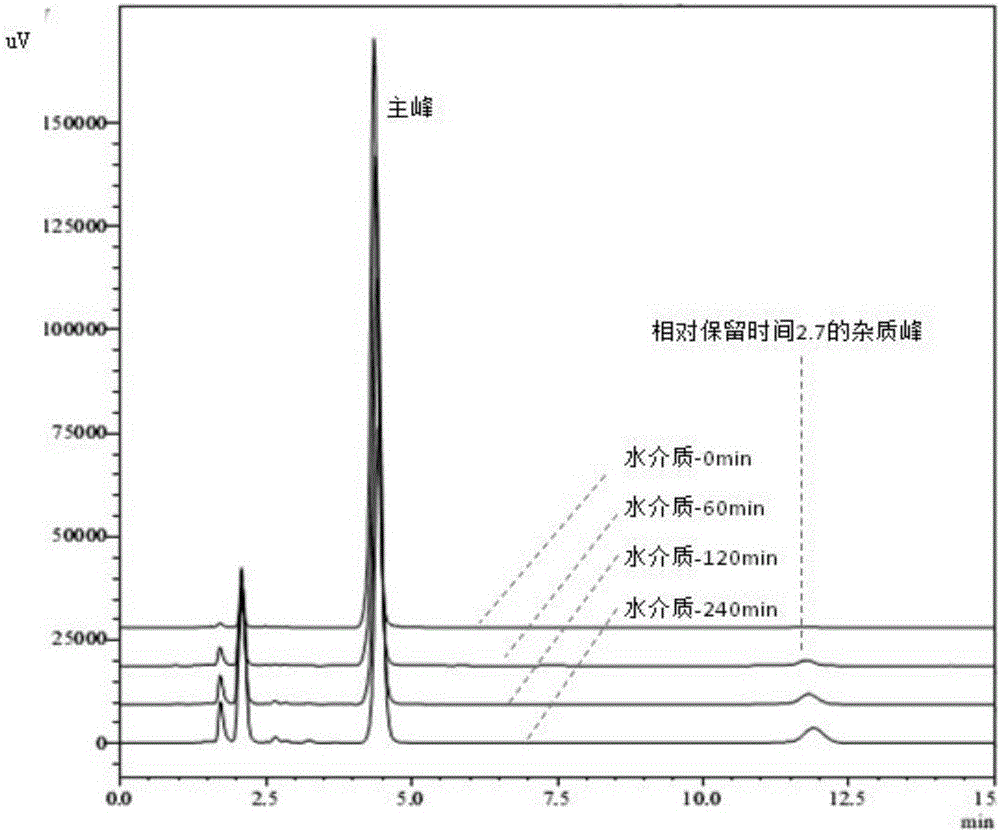
[0051] Example 2. Degradation Law of Esomeprazole Magnesium Enteric-coated Tablets or Enteric-coated Pellet Capsules in Water
[0052] Take the specification of 20mg Esomeprazole magnesium enteric-coated tablets or enteric-coated micropill capsules (remove the capsule shell, take the contents) in a mortar, grind finely, weigh 100mg into a 20ml volumetric flask, and add 0.0125mol / L sodium hydroxide Solution 12mL, ultrasonic 5min, add 7.5ml ethanol and continue ultrasonication for 3min, 0.0125mol / L sodium hydroxide solution to volume, filter, take 2ml of filtrate to 100ml volumetric flask, adjust pH to 7.0±0.05 with 0.1mol / L hydrochloric acid , then dilute to volume with water, place in a water bath at 37°C±0.5°C, sample and filter at 0, 30, 60, 90, 120, 180, 240, 300, and 360 minutes respectively, take 5ml of the filtrate, add 0.25mol / L hydrogen respectively Add 600 μl of sodium oxide, mix well, accurately pipette 20 μl into the liquid chromatograph, and record the chromatogr...
Embodiment 3
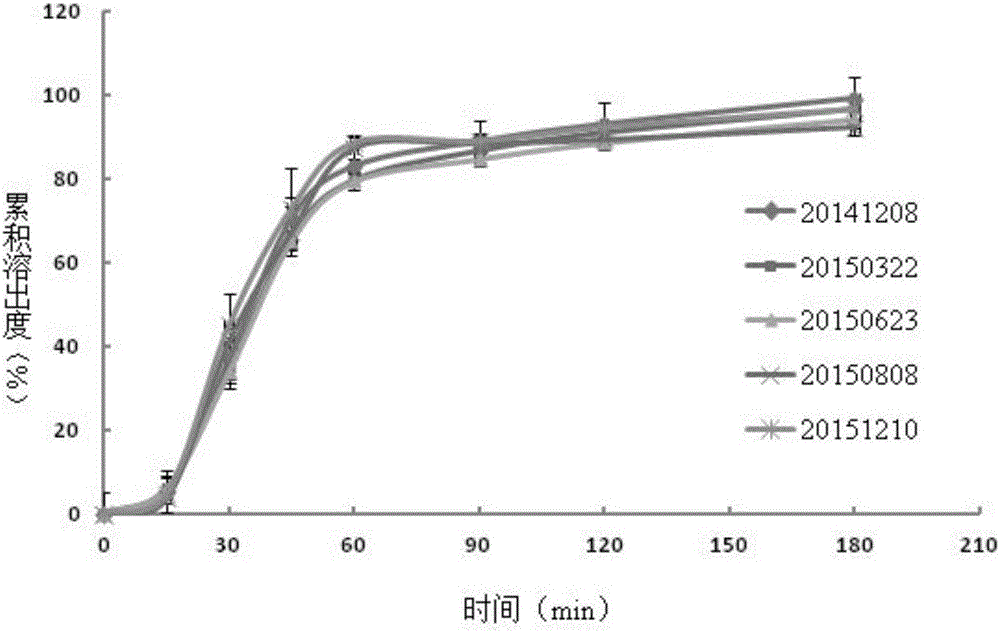
[0061] Example 3. Accuracy (sample recovery) verification of the dissolution rate determination of esomeprazole magnesium enteric-coated tablets
[0062] (1) The recovery rate of sample addition in the phosphate buffer medium of pH 6.0
[0063] Reference substance solution preparation: Weigh about 20 mg of omeprazole, put it in a 100ml measuring bottle, dissolve it with ethanol and dilute to the mark, and use it as the reference substance solution.
[0064] The preparation of need testing solution: get the blank auxiliary material in the esomeprazole magnesium enteric-coated tablet prescription of specification 20mg, grind finely, take by weighing 100mg powder in 100ml volumetric flask, add 0.0125mol / L sodium hydroxide solution, Sonicate for 5min, add 37.5ml of ethanol, sonicate for 3min, and dilute to the mark with 0.0125mol / L sodium hydroxide solution. Filter, take 5ml of continued filtrate and put it in a 100ml volumetric flask, take 9 parts in total, add three parts of re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com