Preparation method of fusion protein for inhibiting clostridium perfringens infection
A fusion protein and protein technology, applied in biochemical equipment and methods, fusion polypeptides, chemical instruments and methods, etc., can solve problems such as difficulty in increasing renaturation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1. Soluble expression of α-β2-ε-his
[0051] 1. Synthetic genes
[0052] The application designed three fusion genes, which are α-β2-ε-hisY gene shown in SEQ ID No.1, α-β2-ε-hisW gene shown in SEQ ID No.3, and SEQ ID No.4 The indicated pmα-β2-ε-hisW gene.
[0053] Both the α-β2-ε-hisY gene and the α-β2-ε-hisW gene encode the protein α-β2-ε-his shown in SEQ ID No.2. The pmα-β2-ε-hisW gene encodes the protein pmα-β2-ε-hisW shown in SEQ ID No.5. α-β2-ε-his is a protein obtained by deleting amino acid residues 52-146, 464-492 and 743-750 of pmα-β2-ε-hisW.
[0054] The α-β2-ε-Y gene shown in the 151-2814th position of SEQ ID No.1 is synthesized by chemical synthesis (the protein shown in the 51-937th amino acid residue of encoding SEQ ID No.2) , the α-β2-ε-W gene shown in the 151-2814th position of SEQ ID No.3 (the protein shown in the 51-937th amino acid residue of encoding SEQ ID No.2), SEQ ID No.4 The pmα-β2-ε-W gene shown at positions 151-3210 of the pmα-β2-...
Embodiment 2
[0075] Embodiment 2, animal immune protective test of α-β2-ε-his
[0076] 1. Preparation of anti-Clostridium perfringens vaccine
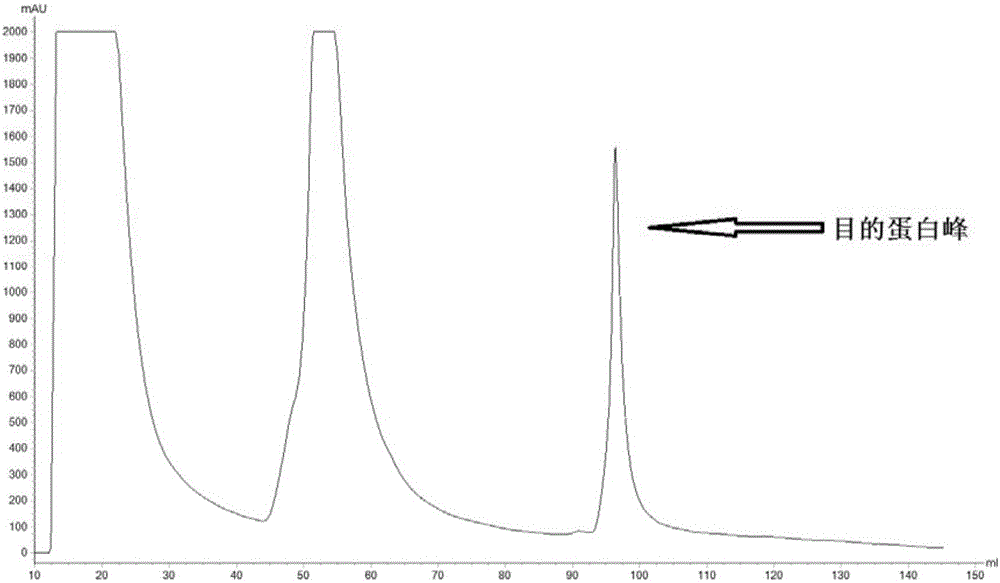
[0077] The α-β2-ε-his protein purified by molecular sieves in Example 1 was dissolved in sterile PBS to obtain an α-β2-ε-his solution with an α-β2-ε-his concentration of 1000 μg / mL for immunization. The α-β2-ε-his solution and Freund's adjuvant were mixed in an equal volume of 1:1, and emulsified to prepare an oil emulsion vaccine, which was named the first vaccine. The α-β2-ε-his solution and incomplete Freund's adjuvant were mixed in equal volumes of 1:1, and emulsified to prepare an oil emulsion vaccine, which was named as the secondary vaccine.
[0078] Take out the A-type Clostridium perfringens virulent strain C57-10, the B-type Clostridium perfringens virulent strain C58-5, and the C-type Clostridium perfringens virulent strain purchased from the China Veterinary Drug Administration. Strain C59-4, Clostridium perfringens type D virulent st...
Embodiment 3
[0101] Example 3, Optimization of α-β2-ε-his Induced Expression Conditions
[0102] 1. Optimization of induction temperature and time
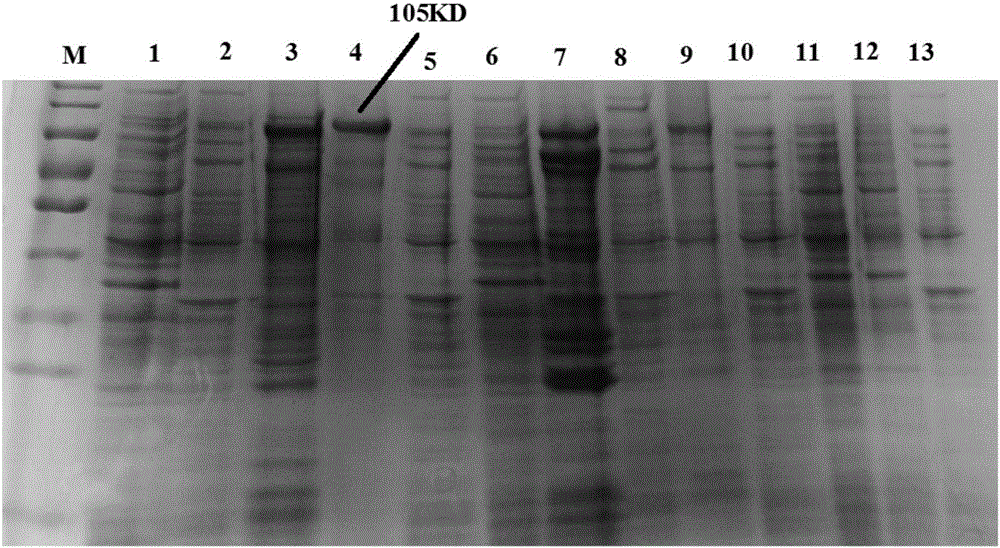
[0103] Inoculate BL21(DE3) / pET30a-α-β2-ε-Y in LB liquid medium containing 50 μg / ml kanamycin (add kanamycin to LB liquid medium until the concentration of kanamycin is 50 μg / ml obtained medium), 37 ° C, using Thermo MaxQ6000 type full-temperature shaker 200 rpm shaking culture to OD 600 When the value (the LB liquid medium containing 50 μg / ml kanamycin was used as the blank control) reached 0.6, isopropylthio-β-D-galactoside (IPTG) was added to induce the following six kinds of expression respectively. The first induced expression was induced with 0.75 mM IPTG for 1 hour at 37°C. The second induced expression was induced with 0.75 mM IPTG for 2 hours at 37°C. The third induced expression was induced with 0.75 mM IPTG for 4 hours at 37°C. The fourth induced expression was induced with 0.75 mM IPTG for 5 hours at 37°C. The fifth induced exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com